Sotatercept analog improves cardiopulmonary remodeling and pulmonary hypertension in experimental left heart failure
- PMID: 36910526
- PMCID: PMC9996114
- DOI: 10.3389/fcvm.2023.1064290
Sotatercept analog improves cardiopulmonary remodeling and pulmonary hypertension in experimental left heart failure
Abstract
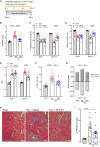
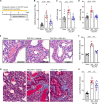
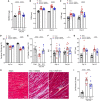
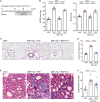
Pulmonary hypertension due to left heart disease (PH-LHD) is the most frequent manifestation of PH but lacks any approved treatment. Activin receptor type IIA-Fc fusion protein (ActRIIA-Fc) was found previously to be efficacious in experimental and human pulmonary arterial hypertension (PAH). Here we tested the hypothesis that ActRIIA-Fc improves pulmonary vascular remodeling and alleviates PH in models of PH-LHD, specifically in subtypes of heart failure with reduced ejection fraction (PH-HFrEF) and preserved ejection fraction (PH-HFpEF). Treatment with murine ActRIIA-Fc reduced cardiac remodeling and improved cardiac function in two mouse models of left heart disease without PH, confirming that this inhibitor of activin-class ligand signaling can exert cardioprotective effects in heart failure. In a mouse model of PH-HFrEF with prolonged pressure overload caused by transverse aortic constriction, ActRIIA-Fc treatment significantly reduced pulmonary vascular remodeling, pulmonary fibrosis, and pulmonary hypertension while exerting beneficial structural, functional, and histological effects on both the left and right heart. Additionally, in an obese ZSF1-SU5416 rat model of PH-HFpEF with metabolic dysregulation, therapeutic treatment with ActRIIA-Fc normalized SMAD3 overactivation in pulmonary vascular and perivascular cells, reversed pathologic pulmonary vascular and cardiac remodeling, improved pulmonary and cardiac fibrosis, alleviated PH, and produced marked functional improvements in both cardiac ventricles. Studies in vitro revealed that treatment with ActRIIA-Fc prevents an abnormal, glucose-induced, activin-mediated, migratory phenotype in human pulmonary artery smooth muscle cells, providing a mechanism by which ActRIIA-Fc could exert therapeutic effects in experimental PH-HFpEF with metabolic dysregulation. Our results demonstrate that ActRIIA-Fc broadly corrects cardiopulmonary structure and function in experimental PH-LHD, including models of PH-HFrEF and PH-HFpEF, leading to alleviation of PH under diverse pathophysiological conditions. These findings highlight the important pathogenic contributions of activin-class ligands in multiple forms of experimental PH and support ongoing clinical evaluation of human ActRIIA-Fc (sotatercept) in patients with PH-HFpEF.
Keywords: ActRIIA-Fc; Group 2 pulmonary hypertension (PH); activin; heart failure with preserved ejection fraction; heart failure with reduced ejection fraction; pulmonary arterial hypertension; smooth muscle cell; vascular remodeling.
Copyright © 2023 Joshi, Atabay, Liu, Ding, Briscoe, Alexander, Andre, Kumar and Li.
Conflict of interest statement
All authors were employed by Acceleron Pharma Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Figures


References
-
- Hoeper M, Humbert M, Souza R, Idrees M, Kawut S, Sliwa-Hahnle K, et al. A global view of pulmonary hypertension. Lancet Respir Med. (2016) 4:306–22. - PubMed
-
- Adler J, Gerhardt F, Wissmüller M, Adler C, Baldus S, Rosenkranz S. Pulmonary hypertension associated with left-sided heart failure. Curr Opin Cardiol. (2020) 35:610–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

