Humoral Effect of SARS-CoV-2 mRNA vaccination with booster dose in solid tumor patients with different anticancer treatments
- PMID: 36910621
- PMCID: PMC9992722
- DOI: 10.3389/fonc.2023.1089944
Humoral Effect of SARS-CoV-2 mRNA vaccination with booster dose in solid tumor patients with different anticancer treatments
Abstract
Introduction: Cancer patients are at risk for serious complications in case of SARS-CoV-2 infection. In these patients SARS-CoV-2 vaccination is strongly recommended, with the preferential use of mRNA vaccines. The antibody response in cancer patients is variable, depending on the type of cancer and antitumoral treatment. In solid tumor patients an antibody response similar to healthy subjects has been confirmed after the second dose. Only few studies explored the duration of immunization after the two doses and the effect of the third dose.
Methods: In our study we explored a cohort of 273 solid tumor patients at different stages and treated with different anticancer therapies.
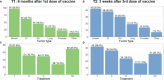
Results and discussion: Our analysis demonstrated that the persistence of the neutralizing antibody and the humoral response after the booster dose of vaccine was not dependent on either the tumor type, the stage or type of anticancer treatment.
Keywords: Comirnaty; IgG; SARS-CoV-2; anticancer treatment; metastasis; neutralizing antibodies; solid tumors.
Copyright © 2023 Piubelli, Valerio, Verzè, Nicolis, Mantoan, Zamboni, Perandin, Rizzi, Tais, Degani, Caldrer, Gobbi, Bisoffi and Gori.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Guarneri V, Bassan F, Zagonel V, Milella M, Zaninelli M, Cattelan AM, et al. Epidemiology and clinical course of severe acute respiratory syndrome coronavirus 2 infection in cancer patients in the veneto oncology network: The rete oncologica veneta covID19 study. Eur J Cancer Oxf Engl 1990. (2021) 147:120–7. doi: 10.1016/j.ejca.2021.01.021 - DOI - PMC - PubMed
-
- Dieci MV, Azzarello G, Zagonel V, Bassan F, Gori S, Aprile G, et al. Clinical profile and mortality of sars-Cov-2 infection in cancer patients across two pandemic time periods (Feb 2020-sep 2020; sep 2020-may 2021) in the veneto oncology network: The ROVID study. Eur J Cancer Oxf Engl 1990. (2022) 167:81–91. doi: 10.1016/j.ejca.2022.03.005 - DOI - PMC - PubMed
-
- Italian Ministry of Health . Vaccinazione anti SARS-CoV2/COVID-19. estensione dell’intervallo tra le due dosi dei vaccini a mRNA (2021). Available at: https://www.trovanorme.salute.gov.it/norme/render-NormsanPdf?anno=2021&c....
-
- Italian Ministry of Health . Trasmissione parere del CTS in merito alla estensione dell’intervallo tra le due dosi dei vaccini a mRNA e alla seconda dose del vaccino vaxzevria (2021). Available at: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&co....
-
- Italian Ministry of Health . Aggiornamento parere CTS vaccini (2021). Available at: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&co....
LinkOut - more resources
Full Text Sources
Miscellaneous

