Operando Studies of Electrochemical Denitrogenation and Its Mitigation of N-Doped Carbon Catalysts in Alkaline Media
- PMID: 36910874
- PMCID: PMC9990068
- DOI: 10.1021/acscatal.2c05590
Operando Studies of Electrochemical Denitrogenation and Its Mitigation of N-Doped Carbon Catalysts in Alkaline Media
Abstract
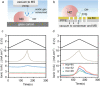
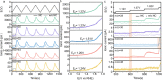
N-doped carbons (NCs) have excellent electrocatalytic performance in oxygen reduction reaction, particularly in alkaline conditions, showing great promise of replacing commercial Pt/C catalysts in fuel cells and metal-air batteries. However, NCs are vulnerable when biased at high potentials, which suffer from denitrogenation and carbon corrosion. Such material degradation drastically undermines the activity, yet its dynamic evolution in response to the applied potentials is challenging to examine experimentally. In this work, we used differential electrochemical mass spectroscopy coupled with an optimized cell and observed the dynamic behaviors of NCs under operando conditions in KOH electrolyte. The corrosion of carbon occurred at ca. 1.2 V vs RHE, which was >0.3 V below the measured onset potential of water oxidation. Denitrogenation proceeded in parallel with carbon corrosion, releasing both NO and NO2. Combined with the ex situ characterizations and density-functional theory calculations, we identified that the pyridinic nitrogen moieties were particularly in peril. Three denitrogenation pathways were also proposed. Finally, we demonstrated that transferring the oxidation reaction sites to the well-deposited metal hydroxide with optimized loading was effective in suppressing the N leaching. This work showed the dynamic evolution of NC under potential bias and might cast light on understanding and mitigating NC deactivation for practical applications.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Xie H.; Xie X. H.; Hu G. X.; Prabhakaran V.; Saha S.; Gonzalez-Lopez L.; Phakatkar A. H.; Hong M.; Wu M. L.; Shahbazian-Yassar R.; Ramani V.; Al-Sheikhly M. I.; Jiang D. E.; Shao Y. Y.; Hu L. B. Ta-TiOx Nanoparticles as Radical Scavengers to Improve the Durability of Fe-N-C Oxygen Reduction Catalysts. Nat. Energy 2022, 7, 281–289. 10.1038/s41560-022-00988-w. - DOI
-
- Tang T.; Jiang W. J.; Liu X. Z.; Deng J.; Niu S.; Wang B.; Jin S. F.; Zhang Q.; Gu L.; Hu J. S.; Wan L. J. Metastable Rock Salt Oxide-Mediated Synthesis of High-Density Dual-Protected M@NC for Long-Life Rechargeable Zinc-Air Batteries with Record Power Density. J. Am. Chem. Soc. 2020, 142, 7116–7127. 10.1021/jacs.0c01349. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
