Real pandemic world results of pharmacokinetic-tailored personalized prophylaxis of bleeds in Polish children and adolescents with severe hemophilia A
- PMID: 36911027
- PMCID: PMC9996297
- DOI: 10.3389/fped.2023.1084539
Real pandemic world results of pharmacokinetic-tailored personalized prophylaxis of bleeds in Polish children and adolescents with severe hemophilia A
Abstract
Introduction: In 2020, the new nationwide protocol of prophylaxis in Polish plasma-derived FVIII (pdFVIII) previously treated patients (PTPs) with severe hemophilia A (sHA) was introduced, resulting in the necessity of switching from pdFVIII to recombinant FVIII (octocog-alpha; rFVIII). The study aimed to: (1) assess the safety of switching from pdFVIII to rFVIII, (2) assess the safety and efficacy of pharmacokinetically based (PK-based) personalized prophylaxis in severe hemophilia A.
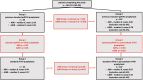
Patients and methods: 151 children and adolescents receiving prophylaxis with a standard dose (40 U/kg 3 x weekly) of pdFVIII were included in this study. Annualized bleeding rate (ABR) and annualized joint bleeding rate (AJBR) were analyzed for all patients before enrollment. Using myPKFiT application, pharmacokinetic (PK) analysis followed by the selection of the optimal model of prophylaxis was performed in all patients. Two possible models of prophylaxis (standard-dose rFVIII versus PK-based rFVIII) were discussed, with parents leaving the choice to their decision. Parents reported all episodes of bleeds. Screening for inhibitor was performed every 3 months. ABR and AJBR were prospectively analyzed again after a minimum follow-up time of 26 weeks.
Results: 141/151 (93.4%) patients completed the study. 34 patients decided to continue standard prophylaxis with rFVIII (Group I), whereas 107 were switched to PK-based prophylaxis (Group II). The risk of inhibitor development could be assessed in 137/151 (90.7%) patients. Only 2/137 (1.47%) patients (both on PK-based prophylaxis) developed low-titer inhibitor with its spontaneous elimination. The retrospective analysis of bleeds during the last 12 months of standard pdFVIII prophylaxis revealed that patients who decided to continue standard prophylaxis had historically lower ABR and AJBR than those who started PK-based personalized prophylaxis. After a minimum of 26 weeks, ABR and AJBR improved significantly in both groups. There was no significant difference in ABR and AJBR between Group I and Group II during the follow-up period. However, the rate of reduction of ABR and AJBR was higher in patients on PK-based personalized prophylaxis.
Conclusion: (1) Switching from pdFVIII to rFVIII (octocog-alpha) in PTPs with sHA is safe, (2) PK-based personalized prophylaxis may decrease ABR and AJBR in children and adolescents with sHA.
Keywords: annualized bleeding rate; annualized joint bleeding rate; bleeding prophylaxis; children; hemophilia A; plasma-derived FVIII; recombinant FVIII.
© 2023 Urasiński, Paczóska, Badowska, Bobrowska, Dakowicz, Dobaczewski, Latos-Grażyńska, Karolczyk, Klukowska, Kołtan, Wojdalska, Łaguna, Niedźwiecki, Pietrys, Radoń-Proskura, Radwańska, Rurańska, Szczepański, Wasiński, Woźnica-Karczmarz, Zielezińska, Królak and Ociepa.
Conflict of interest statement
TU: acted as a consultant, received speaker’s fees and/or research funding from Takeda, NovoNordisk, Roche, Amgen, Phizer, CLS Behring. KP, WB, GD, EL-G, GK, MW, DP, AK, KZ: received speaker fees and reimbursement for attending symposia from Takeda. ŁD, AK, MR, IW-K: received speaker fees and reimbursement for attending symposia from Takeda, Roche, NovoNordisk. AK: received speaker fees and reimbursement for attending symposia from Takeda, Roche, NovoNordisk, CSL Behring, Octapharma, Novartis, JazzPharma, Teva Pharmaceutical. PŁ: received speaker fees and reimbursement for attending symposia from Takeda, Roche, NovoNordisk, CSL Behring, Octapharma. MN: received speaker fees and reimbursement for attending symposia from Takeda, Roche, NovoNordisk, Octapharma. IR: received speaker fees and reimbursement for attending symposia from Takeda, Roche. TS: acted as a consultant, received speaker’s fees and/or research funding from Takeda, Novartis, Sobi, Roche, Amgen, Servier, CLS Behring. DW: received speaker fees and reimbursement for attending symposia from Takeda, Roche, NovoNordisk, CSL Behring. TO: acted as a consultant, received speaker’s fees and/or research funding and reimbursement for attending symposia from Takeda, NovoNordisk, Roche, JazzPharma. HB, JR-P: stated that they had no interests which might be perceived as posing a conflict or bias.
Figures
References
-
- Collins PW, Fischer K, Morfini M, Blanchette VS, Björkman S. International prophylaxis study group pharmacokinetics expert working group. Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. (2011) 17:2–10. 10.1111/j.1365-2516.2010.02370.x - DOI - PubMed
-
- Reininger AJ, Chehadeh HE. The principles of PK-tailored prophylaxis. Hamostaseologie. (2013) 33(Suppl 1):S32–5. PMID: . - PubMed
LinkOut - more resources
Full Text Sources