Disparate Intent-to-Treat Outcomes for Pediatric Liver Transplantation Based on Indication
- PMID: 36911338
- PMCID: PMC9998153
- DOI: 10.1155/2023/2859384
Disparate Intent-to-Treat Outcomes for Pediatric Liver Transplantation Based on Indication
Abstract
Background: The impact of indication for pediatric liver transplantation on waitlist and post-transplant mortality outcomes is well known, but the impact on intent-to-treat outcomes has not been investigated. Intent-to-treat survival analysis is important in this study because it is more comprehensive, combining the transplant outcomes of waitlist mortality, post-transplant mortality, and transplant rate into a single metric to elucidate any disparities in outcomes based on indication.
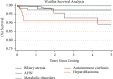
Methods: Cox regression was used to analyze factors impacting survival in 8,002 children listed for liver transplant in the UNOS database between 2006 and 2016. The Kaplan-Meier method and log-rank test were used to assess differences in waitlist, post-transplant, and intent-to-treat mortality among the top 5 indications of biliary atresia, acute hepatic necrosis, metabolic disorders, hepatoblastoma, and autoimmune cirrhosis.
Results: When compared to the reference group of biliary atresia, multivariate analyses showed that every indication was associated with inferior intent-to-treat outcomes except for metabolic disorders. Hepatoblastoma (hazard ratio (HR): 3.73), autoimmune cirrhosis (HR: 1.86), and AHN (HR: 1.77) were associated with significantly increased intent-to-treat mortality. Hepatoblastoma was also associated with increased post-transplant mortality (HR: 3.77) and was the only indication significantly associated with increased waitlist mortality (HR: 6.43).
Conclusion: Significant disparity exists across all indications with respect to an increased intent-to-treat mortality, along with an increased post-transplant and waitlist mortality, when compared to the biliary atresia reference group. If further studies validate these findings, a reexamination of the equitable distribution of allografts for transplant may be warranted as well as a focus on disparities in survival after transplant.
Copyright © 2023 Anna Lang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

