Gut Microbial Metabolites on Host Immune Responses in Health and Disease
- PMID: 36911800
- PMCID: PMC9995988
- DOI: 10.4110/in.2023.23.e6
Gut Microbial Metabolites on Host Immune Responses in Health and Disease
Abstract
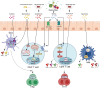
Intestinal microorganisms interact with various immune cells and are involved in gut homeostasis and immune regulation. Although many studies have discussed the roles of the microorganisms themselves, interest in the effector function of their metabolites is increasing. The metabolic processes of these molecules provide important clues to the existence and function of gut microbes. The interrelationship between metabolites and T lymphocytes in particular plays a significant role in adaptive immune functions. Our current review focuses on 3 groups of metabolites: short-chain fatty acids, bile acids metabolites, and polyamines. We collated the findings of several studies on the transformation and production of these metabolites by gut microbes and explained their immunological roles. Specifically, we summarized the reports on changes in mucosal immune homeostasis represented by the Tregs and Th17 cells balance. The relationship between specific metabolites and diseases was also analyzed through latest studies. Thus, this review highlights microbial metabolites as the hidden treasure having potential diagnostic markers and therapeutic targets through a comprehensive understanding of the gut-immune interaction.
Keywords: Bile acids; Immunomodulation; Microbiota; Polyamines; Short-chain fatty acid.
Copyright © 2023. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures

References
-
- Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. - PubMed
-
- van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021;29:700–712. - PubMed
-
- Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. - PMC - PubMed
-
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

