Intrathecal Onasemnogene Abeparvovec for Sitting, Nonambulatory Patients with Spinal Muscular Atrophy: Phase I Ascending-Dose Study (STRONG)
- PMID: 36911944
- PMCID: PMC10200150
- DOI: 10.3233/JND-221560
Intrathecal Onasemnogene Abeparvovec for Sitting, Nonambulatory Patients with Spinal Muscular Atrophy: Phase I Ascending-Dose Study (STRONG)
Abstract
Background: Spinal muscular atrophy (SMA) is a neuromuscular disorder arising from biallelic non-functional survival motor neuron 1 (SMN1) genes with variable copies of partially functional SMN2 gene. Intrathecal onasemnogene abeparvovec administration, at fixed, low doses, may enable treatment of heavier patients ineligible for weight-based intravenous dosing.
Objective: STRONG (NCT03381729) assessed the safety/tolerability and efficacy of intrathecal onasemnogene abeparvovec for sitting, nonambulatory SMA patients.
Methods: Sitting, nonambulatory SMA patients (biallelic SMN1 loss, three SMN2 copies, aged 6-<60 months) received a single dose of intrathecal onasemnogene abeparvovec. Patients were enrolled sequentially into one of three (low, medium, and high) dose cohorts and stratified into two groups by age at dosing: younger (6-<24 months) and older (24-<60 months). Primary endpoints included safety/tolerability, independent standing ≥3 seconds (younger group), and change in Hammersmith Functional Motor Scale Expanded (HFMSE) from baseline (older group) compared with historic controls.
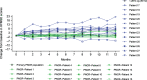
Results: Thirty-two patients were enrolled and completed the study (medium dose, n = 25). All patients had one or more treatment-emergent adverse events, with one serious and related to treatment (transaminase elevations). No deaths were reported. One of 13 patients (7.7%) in the younger group treated with the medium dose achieved independent standing. At Month 12 for the older group receiving the medium dose, change from baseline in HFMSE was significantly improved compared with the SMA historic control population (P < 0.01).
Conclusions: Intrathecal onasemnogene abeparvovec was safe and well-tolerated. Older patients treated with the medium dose demonstrated increases in HFMSE score greater than commonly observed in natural history.
Keywords: Adeno-associated virus; Hammersmith Functional Motor Scale Expanded; clinical trial; gene therapy; intrathecal administration; motor milestones; neurodegenerative disorders; onasemnogene abeparvovec; spinal muscular atrophy; vector genomes.
Conflict of interest statement
RSF has received personal compensation for advisory board participation from Novartis Gene Therapies, Inc., Biogen, Roche, and Scholar Rock, and for consulting from Novartis; editorial fees from Elsevier for co-editing a neurology textbook; license fees from the Children’s Hospital of Philadelphia; and research funding from Novartis Gene Therapies, Inc., Biogen, Roche/Genentech, and Scholar Rock. BTD has served as an
Figures
References
-
- Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al.. Identification and characterization of a spinal muscular atrophy-determining gene, Cell 1995;80(1):155–65. - PubMed
-
- Burghes AHM, McGovern VL Genetics of spinal muscular atrophy. In: Boulis N, O’Connor S, Donsante A, editors.Molecular and cellular therapies for motor neuron diseases. London: Elsevier; 2017, pp. 121–39.
-
- Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, et al.. Correlation between severity and SMN protein level in spinal muscular atrophy, Nat Genet 1997;16(3):265–9. - PubMed
-
- Calucho M, Bernal S, Alías L, March F, Venceslá A, Rodríguez-Álvarez FJ, et al.. Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of reported cases, Neuromuscul Disord 2018;28(3):208–15. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

