Interpreting the Evolutionary Echoes of a Protein Complex Essential for Inner-Ear Mechanosensation
- PMID: 36911992
- PMCID: PMC10089651
- DOI: 10.1093/molbev/msad057
Interpreting the Evolutionary Echoes of a Protein Complex Essential for Inner-Ear Mechanosensation
Abstract
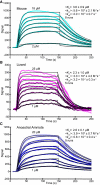
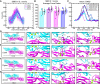
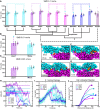
The sensory epithelium of the inner ear, found in all extant lineages of vertebrates, has been subjected to over 500 million years of evolution, resulting in the complex inner ear of modern vertebrates. Inner-ear adaptations are as diverse as the species in which they are found, and such unique anatomical variations have been well studied. However, the evolutionary details of the molecular machinery that is required for hearing are less well known. Two molecules that are essential for hearing in vertebrates are cadherin-23 and protocadherin-15, proteins whose interaction with one another acts as the focal point of force transmission when converting sound waves into electrical signals that the brain can interpret. This "tip-link" interaction exists in every lineage of vertebrates, but little is known about the structure or mechanical properties of these proteins in most non-mammalian lineages. Here, we use various techniques to characterize the evolution of this protein interaction. Results show how evolutionary sequence changes in this complex affect its biophysical properties both in simulations and experiments, with variations in interaction strength and dynamics among extant vertebrate lineages. Evolutionary simulations also characterize how the biophysical properties of the complex in turn constrain its evolution and provide a possible explanation for the increase in deafness-causing mutants observed in cadherin-23 relative to protocadherin-15. Together, these results suggest a general picture of tip-link evolution in which selection acted to modify the tip-link interface, although subsequent neutral evolution combined with varying degrees of purifying selection drove additional diversification in modern tetrapods.
Keywords: cadherins; mechanotransduction; tip link.
© The Author(s) 2023. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Figures

References
-
- Abedin M, King N. 2008. The premetazoan ancestry of cadherins. Science 319:946–948. Available from:http://www.ncbi.nlm.nih.gov/pubmed/18276888. - PubMed
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindah E. 2015. Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25.
-
- Ahmed ZM, Goodyear R, Saima R, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Sheikh R, et al. . 2006. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 26:7022–7034. Available from:http://www.ncbi.nlm.nih.gov/pubmed/16807332. - PMC - PubMed
-
- Assad JA, Shepherd GM, Corey DP. 1991. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7:985–994. Available from:http://www.ncbi.nlm.nih.gov/pubmed/1764247. - PubMed
-
- Bankoff RJ, Jerjos M, Hohman B, Lauterbur ME, Kistler L, Perry GH. 2017. Testing convergent evolution in auditory processing genes between echolocating mammals and the aye-aye, a percussive-foraging primate. Genome Biol Evol. 9:1978–1989. Available from:http://academic.oup.com/gbe/article/9/7/1978/4037174/Testing-Convergent-.... - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

