Specific associations between plasma biomarkers and postmortem amyloid plaque and tau tangle loads
- PMID: 36912178
- PMCID: PMC10165361
- DOI: 10.15252/emmm.202217123
Specific associations between plasma biomarkers and postmortem amyloid plaque and tau tangle loads
Abstract
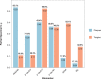
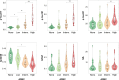
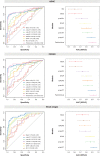
Several promising plasma biomarkers for Alzheimer's disease have been recently developed, but their neuropathological correlates have not yet been fully determined. To investigate and compare independent associations between multiple plasma biomarkers (p-tau181, p-tau217, p-tau231, Aβ42/40, GFAP, and NfL) and neuropathologic measures of amyloid and tau, we included 105 participants from the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND) with antemortem plasma samples and a postmortem neuropathological exam, 48 of whom had longitudinal p-tau217 and p-tau181. When simultaneously including plaque and tangle loads, the Aβ42/40 ratio and p-tau231 were only associated with plaques (ρAβ42/40 [95%CI] = -0.53[-0.65, -0.35], ρp-tau231 [95%CI] = 0.28[0.10, 0.43]), GFAP was only associated with tangles (ρGFAP [95%CI] = 0.39[0.17, 0.57]), and p-tau217 and p-tau181 were associated with both plaques (ρp-tau217 [95%CI] = 0.40[0.21, 0.56], ρp-tau181 [95%CI] = 0.36[0.15, 0.50]) and tangles (ρp-tau217 [95%CI] = 0.52[0.34, 0.66]; ρp-tau181 [95%CI] = 0.36[0.17, 0.52]). A model combining p-tau217 and the Aβ42/40 ratio showed the highest accuracy for predicting the presence of Alzheimer's disease neuropathological change (ADNC, AUC[95%CI] = 0.89[0.82, 0.96]) and plaque load (R2 = 0.55), while p-tau217 alone was optimal for predicting tangle load (R2 = 0.45). Our results suggest that high-performing assays of plasma p-tau217 and Aβ42/40 might be an optimal combination to assess Alzheimer's-related pathology in vivo.
Keywords: Alzheimer's disease; co-pathologies; head-to-head; neuropathology; p-tau species.
© 2023 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Passage Bio, Pinteon Therapeutics, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. OH has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens.
Figures

References
-
- Ashton NJ, Puig‐Pijoan A, Milà‐Alomà M, Fernández‐Lebrero A, García‐Escobar G, González‐Ortiz F, Kac PR, Brum WS, Benedet AL, Lantero‐Rodriguez J et al (2022b) Plasma and CSF biomarkers in a memory clinic: head‐to‐head comparison of phosphorylated tau immunoassays. Alzheimers Dement 10.1002/alz.12841 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

