Clinical outcomes and quality of life after contemporary isolated coronary bypass grafting: a prospective cohort study
- PMID: 36912566
- PMCID: PMC10389413
- DOI: 10.1097/JS9.0000000000000259
Clinical outcomes and quality of life after contemporary isolated coronary bypass grafting: a prospective cohort study
Abstract
Objectives: The objective of the European Multicenter Registry to Assess Outcomes in coronary artery bypass grafting (CABG) patients (DuraGraft Registry) was to determine clinical outcomes and quality of life (QoL) after contemporary CABG that included isolated CABG and combined CABG/valve procedures, using an endothelial damage inhibitor (DuraGraft) intraoperatively for conduit preservation. Here, we report outcomes in the patient cohort undergoing isolated CABG.
Methods: The primary outcome was the composite of all-cause death, myocardial infarction (MI), or repeat revascularization (RR) [major adverse cardiac events (MACE)] at 1 year. Secondary outcomes included the composite of all-cause death, MI, RR, or stroke [major adverse cardiac and cerebrovascular events (MACCE)], and QoL. QoL was assessed with the EuroQol-5 Dimension questionnaire. Independent risk factors for MACE at 1 year were determined using Cox regression analysis.
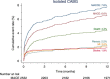
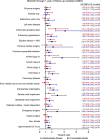
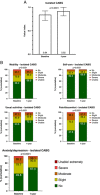
Results: A total of 2532 patients (mean age, 67.4±9.2 years; 82.5% male) underwent isolated CABG. The median EuroScore II was 1.4 [interquartile range (IQR), 0.9-2.3]. MACE and MACCE rates at 1 year were 6.6% and 7.8%, respectively. The rates of all-cause death, MI, RR, and stroke were 4.4, 2.0, 2.2, and 1.9%, respectively. The 30-day mortality rate was 2.3%. Age, extracardiac arteriopathy, left ventricular ejection fraction less than 50%, critical operative state, and left main disease were independent risk factors for MACE. QoL index values improved from 0.84 [IQR, 0.72-0.92] at baseline to 0.92 [IQR, 0.82-1.00] at 1 year ( P <0.0001).
Conclusion: Contemporary European patients undergoing isolated CABG have a low 1-year clinical event rate and an improved QoL.
Trial registration: ClinicalTrials.gov NCT02922088.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
E.C., S.S., M.M., J.A., S.P.S., Y.H.C., and A.B. are members of the Registry Advisory Committee (RAC). L.P.P. is a member of the RAC and is a consultant for Marizyme. MYE is the principal investigator of the registry, the chair of the RAC and a consultant for Marizyme. E.F. received research grants from Somahlution, a Marizyme company. Other authors have nothing to disclose.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures


References
-
- Sousa-Uva M, Neumann FJ, Ahlsson A, et al. . 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4–90. - PubMed
-
- Goldman S, Zadina K, Moritz T, et al. . Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149–56. - PubMed
-
- Caliskan E, de Souza DR, Boning A, et al. . Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat Rev Cardiol 2020;17:155–69. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

