Mechanosensitive pore opening of a prokaryotic voltage-gated sodium channel
- PMID: 36912788
- PMCID: PMC10038658
- DOI: 10.7554/eLife.79271
Mechanosensitive pore opening of a prokaryotic voltage-gated sodium channel
Abstract
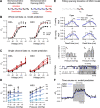
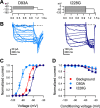
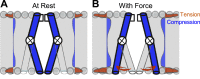
Voltage-gated ion channels (VGICs) orchestrate electrical activities that drive mechanical functions in contractile tissues such as the heart and gut. In turn, contractions change membrane tension and impact ion channels. VGICs are mechanosensitive, but the mechanisms of mechanosensitivity remain poorly understood. Here, we leverage the relative simplicity of NaChBac, a prokaryotic voltage-gated sodium channel from Bacillus halodurans, to investigate mechanosensitivity. In whole-cell experiments on heterologously transfected HEK293 cells, shear stress reversibly altered the kinetic properties of NaChBac and increased its maximum current, comparably to the mechanosensitive eukaryotic sodium channel NaV1.5. In single-channel experiments, patch suction reversibly increased the open probability of a NaChBac mutant with inactivation removed. A simple kinetic mechanism featuring a mechanosensitive pore opening transition explained the overall response to force, whereas an alternative model with mechanosensitive voltage sensor activation diverged from the data. Structural analysis of NaChBac identified a large displacement of the hinged intracellular gate, and mutagenesis near the hinge diminished NaChBac mechanosensitivity, further supporting the proposed mechanism. Our results suggest that NaChBac is overall mechanosensitive due to the mechanosensitivity of a voltage-insensitive gating step associated with the pore opening. This mechanism may apply to eukaryotic VGICs, including NaV1.5.
Keywords: cell biology; electrophysiology; mechanosensitivity; molecular biophysics; patch-clamp; sodium channel; structural biology.
© 2023, Strege et al.
Conflict of interest statement
PS, LC, CA, AM, CA, LM, GF, AB No competing interests declared
Figures










Update of
- doi: 10.1101/2022.05.10.491345
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

