Assessment of the Risk of Venous Thromboembolism in Nonhospitalized Patients With COVID-19
- PMID: 36912838
- PMCID: PMC10011935
- DOI: 10.1001/jamanetworkopen.2023.2338
Assessment of the Risk of Venous Thromboembolism in Nonhospitalized Patients With COVID-19
Abstract
Importance: Patients hospitalized with COVID-19 have higher rates of venous thromboembolism (VTE), but the risk and predictors of VTE among individuals with less severe COVID-19 managed in outpatient settings are less well understood.
Objectives: To assess the risk of VTE among outpatients with COVID-19 and identify independent predictors of VTE.
Design, setting, and participants: A retrospective cohort study was conducted at 2 integrated health care delivery systems in Northern and Southern California. Data for this study were obtained from the Kaiser Permanente Virtual Data Warehouse and electronic health records. Participants included nonhospitalized adults aged 18 years or older with COVID-19 diagnosed between January 1, 2020, and January 31, 2021, with follow-up through February 28, 2021.
Exposures: Patient demographic and clinical characteristics identified from integrated electronic health records.
Main outcomes and measures: The primary outcome was the rate per 100 person-years of diagnosed VTE, which was identified using an algorithm based on encounter diagnosis codes and natural language processing. Multivariable regression using a Fine-Gray subdistribution hazard model was used to identify variables independently associated with VTE risk. Multiple imputation was used to address missing data.
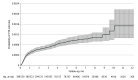
Results: A total of 398 530 outpatients with COVID-19 were identified. The mean (SD) age was 43.8 (15.8) years, 53.7% were women, and 54.3% were of self-reported Hispanic ethnicity. There were 292 (0.1%) VTE events identified over the follow-up period, for an overall rate of 0.26 (95% CI, 0.24-0.30) per 100 person-years. The sharpest increase in VTE risk was observed during the first 30 days after COVID-19 diagnosis (unadjusted rate, 0.58; 95% CI, 0.51-0.67 per 100 person-years vs 0.09; 95% CI, 0.08-0.11 per 100 person-years after 30 days). In multivariable models, the following variables were associated with a higher risk for VTE in the setting of nonhospitalized COVID-19: age 55 to 64 years (HR 1.85 [95% CI, 1.26-2.72]), 65 to 74 years (3.43 [95% CI, 2.18-5.39]), 75 to 84 years (5.46 [95% CI, 3.20-9.34]), greater than or equal to 85 years (6.51 [95% CI, 3.05-13.86]), male gender (1.49 [95% CI, 1.15-1.96]), prior VTE (7.49 [95% CI, 4.29-13.07]), thrombophilia (2.52 [95% CI, 1.04-6.14]), inflammatory bowel disease (2.43 [95% CI, 1.02-5.80]), body mass index 30.0-39.9 (1.57 [95% CI, 1.06-2.34]), and body mass index greater than or equal to 40.0 (3.07 [1.95-4.83]).
Conclusions and relevance: In this cohort study of outpatients with COVID-19, the absolute risk of VTE was low. Several patient-level factors were associated with higher VTE risk; these findings may help identify subsets of patients with COVID-19 who may benefit from more intensive surveillance or VTE preventive strategies.
Conflict of interest statement
Figures

References
-
- World Health Organization. WHO Coronavirus (COVID-19) dashboard. Accessed February 4, 2023. https://covid19.who.int
-
- Spyropoulos AC, Levy JH, Ageno W, et al. ; Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis . Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1859-1865. doi: 10.1111/jth.14929 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

