Evaluation of Antiviral Activity of Gemcitabine Derivatives against Influenza Virus and Severe Acute Respiratory Syndrome Coronavirus 2
- PMID: 36912867
- PMCID: PMC10081574
- DOI: 10.1021/acsinfecdis.3c00034
Evaluation of Antiviral Activity of Gemcitabine Derivatives against Influenza Virus and Severe Acute Respiratory Syndrome Coronavirus 2
Abstract
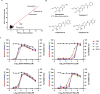
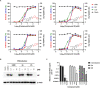
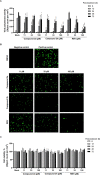
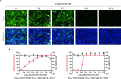
Gemcitabine is a nucleoside analogue of deoxycytidine and has been reported to be a broad-spectrum antiviral agent against both DNA and RNA viruses. Screening of a nucleos(t)ide analogue-focused library identified gemcitabine and its derivatives (compounds 1, 2a, and 3a) blocking influenza virus infection. To improve their antiviral selectivity by reducing cytotoxicity, 14 additional derivatives were synthesized in which the pyridine rings of 2a and 3a were chemically modified. Structure-and-activity and structure-and-toxicity relationship studies demonstrated that compounds 2e and 2h were most potent against influenza A and B viruses but minimally cytotoxic. It is noteworthy that in contrast to cytotoxic gemcitabine, they inhibited viral infection with 90% effective concentrations of 14.5-34.3 and 11.4-15.9 μM, respectively, maintaining viability of mock-infected cells over 90% at 300 μM. Resulting antiviral selectivity was comparable to that of a clinically approved nucleoside analogue, favipiravir. The cell-based viral polymerase assay proved the mode-of-action of 2e and 2h targeting viral RNA replication and/or transcription. In a murine influenza A virus-infection model, intraperitoneal administration of 2h not only reduced viral RNA level in the lungs but also alleviated infection-mediated pulmonary infiltrates. In addition, it inhibited replication of severe acute respiratory syndrome virus 2 infection in human lung cells at subtoxic concentrations. The present study could provide a medicinal chemistry framework for the synthesis of a new class of viral polymerase inhibitors.
Keywords: SARS-CoV-2; antiviral agent; gemcitabine derivatives; influenza virus; polymerase inhibitor.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

