A High-Entropy Oxide as High-Activity Electrocatalyst for Water Oxidation
- PMID: 36913300
- PMCID: PMC10061923
- DOI: 10.1021/acsnano.2c08096
A High-Entropy Oxide as High-Activity Electrocatalyst for Water Oxidation
Abstract
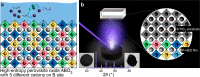
High-entropy materials are an emerging pathway in the development of high-activity (electro)catalysts because of the inherent tunability and coexistence of multiple potential active sites, which may lead to earth-abundant catalyst materials for energy-efficient electrochemical energy storage. In this report, we identify how the multication composition in high-entropy perovskite oxides (HEO) contributes to high catalytic activity for the oxygen evolution reaction (OER), i.e., the key kinetically limiting half-reaction in several electrochemical energy conversion technologies, including green hydrogen generation. We compare the activity of the (001) facet of LaCr0.2Mn0.2Fe0.2Co0.2Ni0.2O3-δ with the parent compounds (single B-site in the ABO3 perovskite). While the single B-site perovskites roughly follow the expected volcano-type activity trends, the HEO clearly outperforms all of its parent compounds with 17 to 680 times higher currents at a fixed overpotential. As all samples were grown as an epitaxial layer, our results indicate an intrinsic composition-function relationship, avoiding the effects of complex geometries or unknown surface composition. In-depth X-ray photoemission studies reveal a synergistic effect of simultaneous oxidation and reduction of different transition metal cations during the adsorption of reaction intermediates. The surprisingly high OER activity demonstrates that HEOs are a highly attractive, earth-abundant material class for high-activity OER electrocatalysts, possibly allowing the activity to be fine-tuned beyond the scaling limits of mono- or bimetallic oxides.
Keywords: green hydrogen; high-entropy oxides; oxygen evolution reaction; perovskite oxide catalysts; scaling reactions; water electrolysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


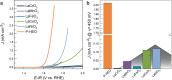

 and OER activity for P-HEO and parent perovskite
oxides. The error bars represent the possible maximum deviation of
the consecutive measurements (top panel) or estimated relative errors
from triplicate CV measurements (bottom panel). Note that the parent
compounds are ordered by
and OER activity for P-HEO and parent perovskite
oxides. The error bars represent the possible maximum deviation of
the consecutive measurements (top panel) or estimated relative errors
from triplicate CV measurements (bottom panel). Note that the parent
compounds are ordered by  rather than atomic number of the TM. (c)
Schematic energy band diagram for LaNiO3, LaCrO3 and P-HEO. The Fermi level (EF) is labeled
by dashed lines in (a) and (c), and the valence band maximum is schematically
shown as linear extrapolation of the leading edge of the VB.
rather than atomic number of the TM. (c)
Schematic energy band diagram for LaNiO3, LaCrO3 and P-HEO. The Fermi level (EF) is labeled
by dashed lines in (a) and (c), and the valence band maximum is schematically
shown as linear extrapolation of the leading edge of the VB.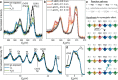
References
-
- Beall C. E.; Fabbri E.; Schmidt T. J. Perovskite Oxide Based Electrodes for the Oxygen Reduction and Evolution Reactions: The Underlying Mechanism. ACS Catal. 2021, 11 (5), 3094–3114. 10.1021/acscatal.0c04473. - DOI
-
- Fabbri E.; Schmidt T. J. Oxygen Evolution Reaction—The Enigma in Water Electrolysis. ACS Catal. 2018, 8 (10), 9765–9774. 10.1021/acscatal.8b02712. - DOI
-
- Man I. C.; Su H.-Y.; Calle-Vallejo F.; Hansen H. A.; Martinez J. I.; Inoglu N. G.; Kitchin J.; Jaramillo T. F.; Nørskov J. K.; Rossmeisl J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem. 2011, 3 (7), 1159–1165. 10.1002/cctc.201000397. - DOI
-
- Rossmeisl J.; Logadottir A.; Nørskov J. K. Electrolysis of Water on (Oxidized) Metal Surfaces. Chem. Phys. 2005, 319 (1–3), 178–184. 10.1016/j.chemphys.2005.05.038. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

