Impact of dispersion media and carrier type on spray-dried proliposome powder formulations loaded with beclomethasone dipropionate for their pulmonary drug delivery via a next generation impactor
- PMID: 36913325
- PMCID: PMC10010524
- DOI: 10.1371/journal.pone.0281860
Impact of dispersion media and carrier type on spray-dried proliposome powder formulations loaded with beclomethasone dipropionate for their pulmonary drug delivery via a next generation impactor
Abstract
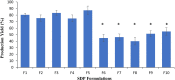
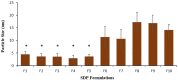
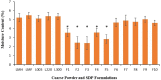
Drug delivery via aerosolization for localized and systemic effect is a non-invasive approach to achieving pulmonary targeting. The aim of this study was to prepare spray-dried proliposome (SDP) powder formulations to produce carrier particles for superior aerosolization performance, assessed via a next generation impactor (NGI) in combination with a dry powder inhaler. SDP powder formulations (F1-F10) were prepared using a spray dryer, employing five different types of lactose carriers (Lactose monohydrate (LMH), lactose microfine (LMF), lactose 003, lactose 220 and lactose 300) and two different dispersion media. The first dispersion medium was comprised of water and ethanol (50:50% v/v ratio), and the second dispersion medium comprised wholly of ethanol (100%). In the first dispersion medium, the lipid phase (consisting of Soya phosphatidylcholine (SPC as phospholipid) and Beclomethasone dipropionate (BDP; model drug) were dissolved in ethanol and the lactose carrier in water, followed by spray drying. Whereas in second dispersion medium, the lipid phase and lactose carrier were dispersed in ethanol only, post spray drying. SDP powder formulations (F1-F5) possessed significantly smaller particles (2.89 ± 1.24-4.48 ± 1.20 μm), when compared to SDP F6-F10 formulations (10.63 ± 3.71-19.27 ± 4.98 μm), irrespective of lactose carrier type via SEM (scanning electron microscopy). Crystallinity of the F6-F10 and amorphicity of F1-F15 formulations were confirmed by XRD (X-ray diffraction). Differences in size and crystallinity were further reflected in production yield, where significantly higher production yield was obtained for F1-F5 (74.87 ± 4.28-87.32 ± 2.42%) then F6-F10 formulations (40.08 ± 5.714-54.98 ± 5.82%), irrespective of carrier type. Negligible differences were noted in terms of entrapment efficiency, when comparing F1-F5 SDP formulations (94.67 ± 8.41-96.35 ± 7.93) to F6-F10 formulations (78.16 ± 9.35-82.95 ± 9.62). Moreover, formulations F1-F5 demonstrated significantly higher fine particle fraction (FPF), fine particle dose (FPD) and respirable fraction (RF) (on average of 30.35%, 890.12 μg and 85.90%) when compared to counterpart SDP powder formulations (F6-F10). This study has demonstrated that when a combination of water and ethanol was employed as dispersion medium (formulations F1-F5), superior formulation properties for pulmonary drug delivery were observed, irrespective of carrier type employed.
Copyright: © 2023 Khan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):600–12. Epub 2003/11/18. doi: 10.1046/j.1365-2125.2003.01893.x ; PubMed Central PMCID: PMC1884297. - DOI - PMC - PubMed
-
- Khan I, Hussein S, Houacine C, Khan Sadozai S, Islam Y, Bnyan R, et al.. Fabrication, characterization and optimization of nanostructured lipid carrier formulations using Beclomethasone dipropionate for pulmonary drug delivery via medical nebulizers. International Journal of Pharmaceutics. 2021;598:120376. doi: 10.1016/j.ijpharm.2021.120376 - DOI - PubMed
-
- Malamatari M, Charisi A, Malamataris S, Kachrimanis K, Nikolakakis I. Spray Drying for the Preparation of Nanoparticle-Based Drug Formulations as Dry Powders for Inhalation. Processes. 2020;8(7):788.
-
- Subramanian S, Khan I, Korale O, Alhnan MA, Ahmed W, Najlah M, et al.. A simple approach to predict the stability of phospholipid vesicles to nebulization without performing aerosolization studies. International Journal of Pharmaceutics. 2016;502(1–2):18–27. doi: 10.1016/j.ijpharm.2016.01.070 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

