An Engineered Probiotic Platform for Cancer Epitope-Independent Targeted Radionuclide Therapy of Solid Tumors
- PMID: 36913614
- PMCID: PMC10497710
- DOI: 10.1002/adhm.202202870
An Engineered Probiotic Platform for Cancer Epitope-Independent Targeted Radionuclide Therapy of Solid Tumors
Abstract
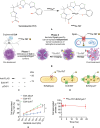
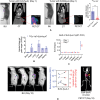
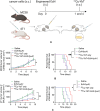
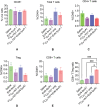
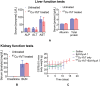
Targeted radionuclide therapy (TRT) is an emerging therapeutic modality for the treatment of various solid cancers. Current approaches rely on the presence of cancer-specific epitopes and receptors against which a radiolabeled ligand is systemically administered to specifically deliver cytotoxic doses of α and β particles to tumors. In this proof-of-concept study, tumor-colonizing Escherichia coli Nissle 1917 (EcN) is utilized to deliver a bacteria-specific radiopharmaceutical to solid tumors in a cancer-epitope independent manner. In this microbe-based pretargeted approach, the siderophore-mediated metal uptake pathway is leveraged to selectively concentrate copper radioisotopes, 64 Cu and 67 Cu, complexed to yersiniabactin (YbT) in the genetically modified bacteria. 64 Cu-YbT facilitates positron emission tomography (PET) imaging of the intratumoral bacteria, whereas 67 Cu-YbT delivers a cytotoxic dose to the surrounding cancer cells. PET imaging with 64 Cu-YbT reveals persistence and sustained growth of the bioengineered microbes in the tumor microenvironment. Survival studies with 67 Cu-YbT reveals significant attenuation of tumor growth and extends survival of both MC38 and 4T1 tumor-bearing mice harboring the microbes. Tumor response to this pretargeted approach correlates with promising anti-tumor immunity, with noticeable CD8+ T:Treg cell ratio. Their strategy offers a pathway to target and ablate multiple solid tumors independent of their epitope and receptor phenotype.
Keywords: cancer theranostics; engineered bacteria; positron emission tomography imaging; pretargeting; siderophore.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
N.A.S. and N.K. have filed a provisional patent application with the US Patent and Trademark Office related to this work.
Figures
References
-
- Imhof A., Brunner P., Marincek N., Briel M., Schindler C., Rasch H., Mäcke H. R., Rochlitz C., Müller‐Brand J., Walter M. A., J. Clin. Oncol. 2011, 29, 2416. - PubMed
-
- Strosberg J., El‐Haddad G., Wolin E., Hendifar A., Yao J., Chasen B., Mittra E., Kunz P. L., Kulke M. H., Jacene H., Bushnell D., O'dorisio T. M., Baum R. P., Kulkarni H. R., Caplin M., Lebtahi R., Hobday T., Delpassand E., Van Cutsem E., Benson A., Srirajaskanthan R., Pavel M., Mora J., Berlin J., Grande E., Reed N., Seregni E., Öberg K., Sierra M. L., Santoro P., et al., N. Engl. J. Med. 2017, 376, 125. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

