Ultrasound-assisted Peptide Nucleic Acids synthesis (US-PNAS)
- PMID: 36913782
- PMCID: PMC10024050
- DOI: 10.1016/j.ultsonch.2023.106360
Ultrasound-assisted Peptide Nucleic Acids synthesis (US-PNAS)
Abstract
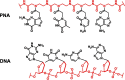
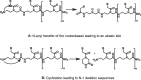
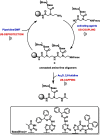
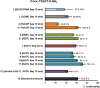
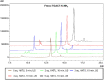
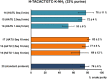
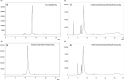
Herein, we developed an innovative and easily accessible solid-phase synthetic protocol for Peptide Nucleic Acid (PNA) oligomers by systematically investigating the ultrasonication effects in all steps of the PNA synthesis (US-PNAS). When compared with standard protocols, the application of the so-obtained US-PNAS approach succeeded in improving the crude product purities and the isolated yields of different PNA, including small or medium-sized oligomers (5-mer and 9-mer), complex purine-rich sequences (like a 5-mer Guanine homoligomer and the telomeric sequence TEL-13) and longer oligomers (such as the 18-mer anti-IVS2-654 PNA and the 23-mer anti-mRNA 155 PNA). Noteworthy, our ultrasound-assisted strategy is compatible with the commercially available PNA monomers and well-established coupling reagents and only requires the use of an ultrasonic bath, which is a simple equipment generally available in most synthetic laboratories.
Keywords: Coupling Conditions; Peptide Nucleic Acid; Solid-Phase Synthesis; Sonochemistry; Ultrasonication.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

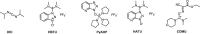



References
-
- S. Siddiquee, K. Rovina A. Azriah, Review of Peptide Nucleic Acid Adv. Technol. Bio. Med. 03 (2015) 1−10 10.4172/2379-1764.1000131. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

