Duration of viral infectiousness and correlation with symptoms and diagnostic testing in non-hospitalized adults during acute SARS-CoV-2 infection: A longitudinal cohort study
- PMID: 36913789
- PMCID: PMC9981266
- DOI: 10.1016/j.jcv.2023.105420
Duration of viral infectiousness and correlation with symptoms and diagnostic testing in non-hospitalized adults during acute SARS-CoV-2 infection: A longitudinal cohort study
Abstract
Background: Guidelines for SARS-CoV-2 have relied on limited data on duration of viral infectiousness and correlation with COVID-19 symptoms and diagnostic testing.
Methods: We enrolled ambulatory adults with acute SARS-CoV-2 infection and performed serial measurements of COVID-19 symptoms, nasal swab viral RNA, nucleocapsid (N) and spike (S) antigens, and replication-competent SARS-CoV-2 by viral growth in culture. We determined average time from symptom onset to a first negative test result and estimated risk of infectiousness, as defined by positive viral growth in culture.
Results: Among 95 adults, median [interquartile range] time from symptom onset to first negative test result was 9 [5] days, 13 [6] days, 11 [4] days, and >19 days for S antigen, N antigen, culture growth, and viral RNA by RT-PCR, respectively. Beyond two weeks, virus growth and N antigen titers were rarely positive, while viral RNA remained detectable among half (26/51) of participants tested 21-30 days after symptom onset. Between 6-10 days from symptom onset, N antigen was strongly associated with culture positivity (relative risk=7.61, 95% CI: 3.01-19.22), whereas neither viral RNA nor symptoms were associated with culture positivity. During the 14 days following symptom onset, the presence of N antigen remained strongly associated (adjusted relative risk=7.66, 95% CI: 3.96-14.82) with culture positivity, regardless of COVID-19 symptoms.
Conclusions: Most adults have replication-competent SARS-CoV-2 for 10-14 after symptom onset. N antigen testing is a strong predictor of viral infectiousness and may be a more suitable biomarker, rather than absence of symptoms or viral RNA, to discontinue isolation within two weeks from symptom onset.
Keywords: COVID-19; Infectiousness; Isolation; SARS-CoV-2; Transmission.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
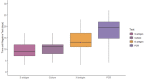
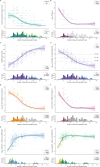
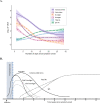
References
-
- World Health Organization. WHO coronavirus (COVID-19) dashboard (2022); https://COVID19.who.int.
-
- World Health Organization . World Health Organization; Geneva: 2021. Technical Specifications For Selection of Essential in Vitro Diagnostics For SARS-CoV-2.
-
- World Health Organization . Interim Guidance; 2020. Laboratory Testing Strategy Recommendations For COVID-19.https://apps.who.int/iris/bitstream/handle/10665/331509/WHO-COVID-19-lab... 21 MarchRef: WHO/2019-nCoV/lab_testing/2020.1. Note: “The role of rapid disposable tests for antigen detection for COVID-19 needs to be evaluated and is not currently recommended for clinical diagnosis pending more evidence on test performance and operational utility. WHO will update this guidance as more information laboratory tests for COVID-19 becomes available.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

