Long-term COVID-19 booster effectiveness by infection history and clinical vulnerability and immune imprinting: a retrospective population-based cohort study
- PMID: 36913963
- PMCID: PMC10079373
- DOI: 10.1016/S1473-3099(23)00058-0
Long-term COVID-19 booster effectiveness by infection history and clinical vulnerability and immune imprinting: a retrospective population-based cohort study
Abstract
Background: Long-term effectiveness of COVID-19 mRNA boosters in populations with different previous infection histories and clinical vulnerability profiles is inadequately understood. We aimed to investigate the effectiveness of a booster (third dose) vaccination against SARS-CoV-2 infection and against severe, critical, or fatal COVID-19, relative to that of primary-series (two-dose) vaccination over a follow-up duration of 1 year.
Methods: This observational, matched, retrospective, cohort study was done on the population of Qatar in people with different immune histories and different clinical vulnerability to infection. The source of data are Qatar's national databases for COVID-19 laboratory testing, vaccination, hospitalisation, and death. Associations were estimated using inverse-probability-weighted Cox proportional-hazards regression models. The primary outcome of the study is the effectiveness of COVID-19 mRNA boosters against infection and against severe COVID-19.
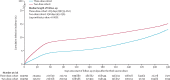
Findings: Data were obtained for 2 228 686 people who had received at least two vaccine doses starting from Jan 5, 2021, of whom 658 947 (29·6%) went on to receive a third dose before data cutoff on Oct 12, 2022. There were 20 528 incident infections in the three-dose cohort and 30 771 infections in the two-dose cohort. Booster effectiveness relative to primary series was 26·2% (95% CI 23·6-28·6) against infection and 75·1% (40·2-89·6) against severe, critical, or fatal COVID-19, during 1-year follow-up after the booster. Among people clinically vulnerable to severe COVID-19, effectiveness was 34·2% (27·0-40·6) against infection and 76·6% (34·5-91·7) against severe, critical, or fatal COVID-19. Effectiveness against infection was highest at 61·4% (60·2-62·6) in the first month after the booster but waned thereafter and was modest at only 15·5% (8·3-22·2) by the sixth month. In the seventh month and thereafter, coincident with BA.4/BA.5 and BA.2·75* subvariant incidence, effectiveness was progressively negative albeit with wide CIs. Similar patterns of protection were observed irrespective of previous infection status, clinical vulnerability, or type of vaccine (BNT162b2 vs mRNA-1273).
Interpretation: Protection against omicron infection waned after the booster, and eventually suggested a possibility for negative immune imprinting. However, boosters substantially reduced infection and severe COVID-19, particularly among individuals who were clinically vulnerable, affirming the public health value of booster vaccination.
Funding: The Biomedical Research Program and the Biostatistics, Epidemiology, and the Biomathematics Research Core (both at Weill Cornell Medicine-Qatar), Ministry of Public Health, Hamad Medical Corporation, Sidra Medicine, Qatar Genome Programme, and Qatar University Biomedical Research Center.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests AAB has received institutional grant funding from Gilead Sciences unrelated to the work presented in this paper. All other authors declare no competing interests.
Figures


Comment in
-
The ups and downs of observational vaccine research.Lancet Infect Dis. 2023 Jul;23(7):767-768. doi: 10.1016/S1473-3099(23)00119-6. Epub 2023 Mar 10. Lancet Infect Dis. 2023. PMID: 36913962 Free PMC article. No abstract available.
References
-
- Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness and durability of BNT162b2 vaccine against hospital and emergency department admissions due to SARS-CoV-2 omicron sub-lineages BA.1 and BA.2 in a large health system in the USA: a test-negative, case-control study. Lancet Respir Med. 2023;11:176–187. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

