TRAK adaptors regulate the recruitment and activation of dynein and kinesin in mitochondrial transport
- PMID: 36914620
- PMCID: PMC10011603
- DOI: 10.1038/s41467-023-36945-8
TRAK adaptors regulate the recruitment and activation of dynein and kinesin in mitochondrial transport
Abstract
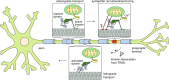
Mitochondrial transport along microtubules is mediated by Miro1 and TRAK adaptors that recruit kinesin-1 and dynein-dynactin. To understand how these opposing motors are regulated during mitochondrial transport, we reconstitute the bidirectional transport of Miro1/TRAK along microtubules in vitro. We show that the coiled-coil domain of TRAK activates dynein-dynactin and enhances the motility of kinesin-1 activated by its cofactor MAP7. We find that TRAK adaptors that recruit both motors move towards kinesin-1's direction, whereas kinesin-1 is excluded from binding TRAK transported by dynein-dynactin, avoiding motor tug-of-war. We also test the predictions of the models that explain how mitochondrial transport stalls in regions with elevated Ca2+. Transport of Miro1/TRAK by kinesin-1 is not affected by Ca2+. Instead, we demonstrate that the microtubule docking protein syntaphilin induces resistive forces that stall kinesin-1 and dynein-driven motility. Our results suggest that mitochondrial transport stalls by Ca2+-mediated recruitment of syntaphilin to the mitochondrial membrane, not by disruption of the transport machinery.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

