Regulated cell death pathways in cardiomyopathy
- PMID: 36914852
- PMCID: PMC10374591
- DOI: 10.1038/s41401-023-01068-9
Regulated cell death pathways in cardiomyopathy
Abstract
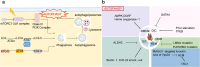
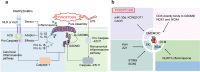
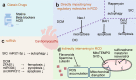
Heart disease is a worldwide health menace. Both intractable primary and secondary cardiomyopathies contribute to malignant cardiac dysfunction and mortality. One of the key cellular processes associated with cardiomyopathy is cardiomyocyte death. Cardiomyocytes are terminally differentiated cells with very limited regenerative capacity. Various insults can lead to irreversible damage of cardiomyocytes, contributing to progression of cardiac dysfunction. Accumulating evidence indicates that majority of cardiomyocyte death is executed by regulating molecular pathways, including apoptosis, ferroptosis, autophagy, pyroptosis, and necroptosis. Importantly, these forms of regulated cell death (RCD) are cardinal features in the pathogenesis of various cardiomyopathies, including dilated cardiomyopathy, diabetic cardiomyopathy, sepsis-induced cardiomyopathy, and drug-induced cardiomyopathy. The relevance between abnormity of RCD with adverse outcome of cardiomyopathy has been unequivocally evident. Therefore, there is an urgent need to uncover the molecular and cellular mechanisms for RCD in order to better understand the pathogenesis of cardiomyopathies. In this review, we summarize the latest progress from studies on RCD pathways in cardiomyocytes in context of the pathogenesis of cardiomyopathies, with particular emphasis on apoptosis, necroptosis, ferroptosis, autophagy, and pyroptosis. We also elaborate the crosstalk among various forms of RCD in pathologically stressed myocardium and the prospects of therapeutic applications targeted to various cell death pathways.
Keywords: cardiomyopathy; cell death; cell signaling.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–79. doi: 10.1093/eurheartj/ehu284. - DOI - PubMed

