Data collection systems for active safety surveillance of vaccines during pregnancy in low- and middle-income countries: developing and piloting an assessment tool (VPASS)
- PMID: 36915061
- PMCID: PMC10010225
- DOI: 10.1186/s12884-023-05417-8
Data collection systems for active safety surveillance of vaccines during pregnancy in low- and middle-income countries: developing and piloting an assessment tool (VPASS)
Abstract
Background: There is an urgent need for active safety surveillance to monitor vaccine exposure during pregnancy in low- and middle-income countries (LMICs). Existing maternal, newborn, and child health (MNCH) data collection systems could serve as platforms for post-marketing active surveillance of maternal immunization safety. To identify sites using existing systems, a thorough assessment should be conducted. Therefore, this study had the objectives to first develop an assessment tool and then to pilot this tool in sites using MNCH data collection systems through virtual informant interviews.
Methods: We conducted a rapid review of the literature to identify frameworks on population health or post-marketing drug surveillance. Four frameworks that met the eligibility criteria were identified and served to develop an assessment tool capable of evaluating sites that could support active monitoring of vaccine safety during pregnancy. We conducted semi-structured interviews in six geographical sites using MNCH data collection systems (DHIS2, INDEPTH, and GNMNHR) to pilot domains included in the assessment tool.
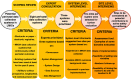
Results: We developed and piloted the "VPASS (Vaccines during Pregnancy - sites supporting Active Safety Surveillance) assessment tool" through interviews with nine stakeholders, including central-level systems key informants and site-level managers from DHIS2 and GNMNHR; DHIS2 in Kampala (Uganda) and Kigali (Rwanda); GNMNHR from Belagavi (India) and Lusaka (Zambia); and INDEPTH from Nanoro (Burkina Faso) and Manhica (Mozambique). The tool includes different domains such as the system's purpose, the scale of implementation, data capture and confidentiality, type of data collected, the capability of integration with other platforms, data management policies and data quality monitoring. Similarities among sites were found regarding some domains, such as data confidentiality, data management policies, and data quality monitoring. Four of the six sites met some domains to be eligible as potential sites for active surveillance of vaccinations during pregnancy, such as a routine collection of MNCH individual data and the capability of electronically integrating individual MNCH outcomes with information related to vaccine exposure during pregnancy. Those sites were: Rwanda (DHIS2), Manhica (IN-DEPTH), Lusaka (GNMNHR), and Belagavi (GNMNHR).
Conclusion: This study's findings should inform the successful implementation of active safety surveillance of vaccines during pregnancy by identifying and using active individual MNCH data collection systems in LMICs.
Keywords: COVID-19; DHIS2; Data collection system; Global Network; INDEPTH; Maternal immunization; Pharmacovigilance; Pregnancy; Safety surveillance; Vaccines.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Maternal Immunization Safety Monitoring in Low- and Middle-Income Countries: A Roadmap for Program Development. MaternalImmunizationSafetyMonitoringInLMICs.pdf [Internet]. 2017 [cited 2022 Mar 12]. Available from: https://www.gapps.org/PDF/MaternalImmunizationSafetyMonitoringInLMICs.pdf
-
- Sobanjo-TerMeulen A, Munoz FM, Kaslow DC, Klugman KP, Omer SB, Vora P, et al. Maternal interventions vigilance harmonization in low- and middle-income countries: Stakeholder meeting report; Amsterdam, May 1–2, 2018. Vaccine. 2019;37(20):2643–50. doi: 10.1016/j.vaccine.2019.03.060. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous