Safety and tolerability of Sacubitril/Valsartan in heart failure patient with reduced ejection fraction
- PMID: 36915075
- PMCID: PMC10012729
- DOI: 10.1186/s12872-023-03070-9
Safety and tolerability of Sacubitril/Valsartan in heart failure patient with reduced ejection fraction
Abstract
Background: Angiotensin receptor blocker and a neprilysin inhibitor (ARNI) has emerged as an innovative therapy for patients of heart failure with reduced ejection fraction (HFrEF). The purpose of this study was to assess the safety and tolerability of Sacubitril/Valsartan in patient with HFrEF in Pakistani population.
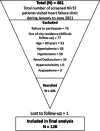
Methods: This proof-of-concept, open label non-randomized clinical trial was conducted at a tertiary care cardiac center of Karachi, Pakistan. Patients with HFrEF were prescribed with Sacubitril/Valsartan and followed for 12 weeks for the assessment of safety and tolerability. Safety measures included incidence of hypotension, renal dysfunction, hyperkalemia, and angioedema.
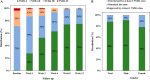
Results: Among the 120 HFrEF patients, majority were male (79.2%) with means age of 52.73 ± 12.23 years. At the end of 12 weeks, four (3.3%) patients died and eight (6.7%) dropped out of the study. In the remaining 108 patients, 80.6% (87) of the patients were tolerant to the prescribed dose. Functional class improved gradually with 75.0% (81) in class I and 24.1% (26) in class II, and only one (0.9%) patient in class III at the end of 12 weeks. Hyperkalemia remains the main safety concern with incidence rate of 21.3% (23) followed by hypotension in 19.4% (21), and renal dysfunction in 3.7% (4) of the patients.
Conclusions: Sacubitril/Valsartan therapy in HFrEF patients is safe and moderately tolerated among the Pakistani population. It can be used as first line of treatment for these patients.
Trial registration: NCT05387967. Registered 24 May 2022-Retrospectively registered, https://clinicaltrials.gov/ct2/show/NCT05387967.
Keywords: Heart failure; Reduced ejection fraction; Sacubitril; Safety and tolerability.
© 2023. The Author(s).
Conflict of interest statement
The Medical Affairs division of Getz Pharma Pvt. Ltd. provided financial support for data collection and manuscript drafting, however, they have no role in interpretation and presentation of data. The content is solely the responsibility of the authors and all authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical