PROphylaxis for paTiEnts at risk of COVID-19 infecTion (PROTECT-V)
- PMID: 36915199
- PMCID: PMC10009350
- DOI: 10.1186/s13063-023-07128-z
PROphylaxis for paTiEnts at risk of COVID-19 infecTion (PROTECT-V)
Abstract
Background: Despite the introduction of vaccination, there remains a need for pre-exposure prophylactic agents against SARS-CoV-2. Several patient groups are more vulnerable to SARS-CoV-2 infection by virtue of underlying health conditions, treatments received or suboptimal responses to vaccination.
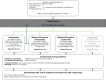
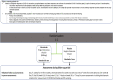
Methods: PROTECT-V is a platform trial testing pre-exposure prophylactic interventions against SARS-CoV-2 infection in vulnerable patient populations (organ transplant recipients; individuals with oncological/haematological diagnoses, immune deficiency or autoimmune diseases requiring immunosuppression or on dialysis). Multiple agents can be evaluated across multiple vulnerable populations sharing placebo groups, with the option of adding additional treatments at later time points as these become available. The primary endpoint is symptomatic SARS-CoV-2 infection, and each agent will be independently evaluated in real time when the required number of events occurs. Presently, three agents are approved in the platform: intranasal niclosamide, nasal and inhaled ciclesonide and intravenous sotrovimab.
Discussion: Despite the introduction of vaccination, there remains a need for pre-exposure prophylactic agents against SARS-CoV-2. Several patient groups are more vulnerable to COVID-19 disease by virtue of underlying health conditions, treatments received or suboptimal responses to vaccination.
Trial registration: ClinicalTrials.gov NCT04870333. EudraCT 2020-004144-28.
Keywords: COVID-19; Ciclesonide; Niclosamide; Platform; Prophylaxis; SARS-CoV-2; Sotrovimab; Trial.
© 2023. The Author(s).
Conflict of interest statement
TJLH has received research funding from AstraZeneca outside of the submitted work. RS receives research funding from UNION Therapeutics and GSK. DD and MCX receive research funding from GSK. DD has also received honoraria from AstraZeneca, Boehringer Ingelheim and Gilead Sciences. TFH is an employee of GSK (but has no involvement in the sotrovimab arm of the study). TFH designed the core protocol and niclosamide arm of the trial with RS. VJ has received grant funding from GSK, Baxter Healthcare and Biocon and honoraria from Bayer, AstraZeneca, Boehringer Ingelheim, NephroPlus and Zydus Cadilla, under the policy of all honoraria being paid to the organisation.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous