Roles of neuropathology-associated reactive astrocytes: a systematic review
- PMID: 36915214
- PMCID: PMC10009953
- DOI: 10.1186/s40478-023-01526-9
Roles of neuropathology-associated reactive astrocytes: a systematic review
Abstract
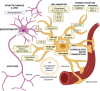
In the contexts of aging, injury, or neuroinflammation, activated microglia signaling with TNF-α, IL-1α, and C1q induces a neurotoxic astrocytic phenotype, classified as A1, A1-like, or neuroinflammatory reactive astrocytes. In contrast to typical astrocytes, which promote neuronal survival, support synapses, and maintain blood-brain barrier integrity, these reactive astrocytes downregulate supportive functions and begin to secrete neurotoxic factors, complement components like C3, and chemokines like CXCL10, which may facilitate recruitment of immune cells across the BBB into the CNS. The proportion of pro-inflammatory reactive astrocytes increases with age through associated microglia activation, and these pro-inflammatory reactive astrocytes are particularly abundant in neurodegenerative disorders. As the identification of astrocyte phenotypes progress, their molecular and cellular effects are characterized in a growing array of neuropathologies.
Keywords: A1 astrocytes; Activated astrocytes; Neurodegeneration; Neuroinflammation; Neurotoxic reactive astrocytes.
© 2023. The Author(s).
Conflict of interest statement
Not applicable.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

