Antioxidants prevent particulate matter-induced senescence of lung fibroblasts
- PMID: 36915477
- PMCID: PMC10006845
- DOI: 10.1016/j.heliyon.2023.e14179
Antioxidants prevent particulate matter-induced senescence of lung fibroblasts
Abstract
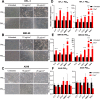
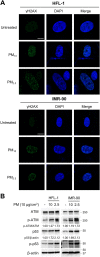
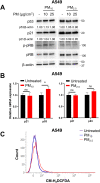
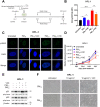
Particulate matter (PM) contributes to human diseases, particularly lung disease; however, the molecular mechanism of its action is yet to be determined. Herein, we found that prolonged PM exposure induced the cellular senescence of normal lung fibroblasts via a DNA damage-mediated response. This PM-induced senescence (PM-IS) was only observed in lung fibroblasts but not in A549 lung adenocarcinoma cells. Mechanistic analysis revealed that reactive oxygen species (ROS) activate the DNA damage response signaling axis, increasing p53 phosphorylation, ultimately leading to cellular senescence via an increase in p21 expression without affecting the p16-pRB pathway. A549 cells, instead, were resistant to PM-IS due to the PM-induced ROS production suppression. Water-soluble antioxidants, such as vitamin C and N-Acetyl Cysteine, were found to alleviate PM-IS by suppressing ROS production, implying that antioxidants are a promising therapeutic intervention for PM-mediated lung pathogenesis.
Keywords: Antioxidants; Cellular senescence; DNA damage Response; Particulate matter; Reactive oxygen species.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Winterbottom C.J., Shah R.J., Patterson K.C., Kreider M.E., Panettieri R.A., Jr., Rivera-Lebron B., Miller W.T., Litzky L.A., Penning T.M., Heinlen K., Jackson T., Localio A.R., Christie J.D. Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest. 2018;153(5):1221–1228. doi: 10.1016/j.chest.2017.07.034. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

