Validation of a Modified National Eye Institute Grading Scale for Corneal Fluorescein Staining
- PMID: 36915716
- PMCID: PMC10007867
- DOI: 10.2147/OPTH.S398843
Validation of a Modified National Eye Institute Grading Scale for Corneal Fluorescein Staining
Abstract
Purpose: Validation of the novel Lexitas modified NEI scale for use in assessment of corneal fluorescein staining.
Patients and methods: A series of 18 illustrations and 14 clinical photographs depicting varying severity levels of corneal fluorescein staining were assessed by 3 independent examiners. Regions of the cornea were graded for staining severity based on 3 different grading scales: the original NEI staining scale (density-based scoring; 0-3 scale), a structured version of the NEI scale (dot-count scoring; 0-3 scale), and the Lexitas modified NEI staining scale (0-4 scale with half-point increments). Kappa statistics (simple and weighted) were computed to determine intra-examiner image grading repeatability for each examiner over 2 separate assessments. Inter-examiner assessment reliability utilized the scores from the first read of each examiner, and pairs of examiners to compute kappa statistics.
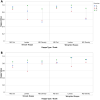
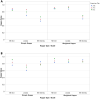
Results: Data was analyzed from the scores provided by the examiners from each gradable corneal region on 32 images (18 illustrations and 14 photographs) for a total of 154 corneal regions across the 3 grading scales for each validation run. The mean intra-examiner simple/weighted kappa values using the NEI density, NEI dot count, and the Lexitas modified NEI staining scales were 0.67/0.72, 0.91/0.94, 0.80/0.92 for the graded illustrations, and 0.83/0.88, 0.76/0.85, 0.77/0.88 for the graded photographs, respectively. The mean inter-examiner simple/weighted kappa values using the NEI density, NEI dot count, and the Lexitas modified NEI staining scales were 0.59/0.65, 0.86/0.90, and 0.78/0.91 for the graded illustrations, and 0.80/0.88, 0.84/0.89, 0.69/0.88 for the graded photographs, respectively.
Conclusion: The expanded scale of the Lexitas modified NEI staining scale demonstrated a high degree of reliability and repeatability of grading assessments within and across individual examiners, comparing favorably with the original NEI staining scale. A future investigation into the in-office utility of the Lexitas modified NEI staining scale is warranted.
Keywords: clinical trials; corneal sodium fluorescein staining; dry eye disease; repeatability; validation.
© 2023 Sall et al.
Conflict of interest statement
AD Pucker, RC Zink, and G Magrath are employees of Lexitas Pharma Services, KL Ice is a co-founder and former employee of Lexitas Pharma Services. K Sall and GN Foulks are consultants to Lexitas Pharma Services. AD Pucker has received research or consulting support from Alcon, Art Optical, CopperVision, Euclid Systems, Kala, and Nevakar over the past three years, though this work is unrelated to the current project. K Sall has received consulting support from Allysta, Cloudbreak, Famy, Tarsier, Biorasi, Premark, Betaliq, Lexitas and Glaukos, although this work is unrelated to the current project. GN Foulks has received consulting support from Allysta, Aramis, Bristol Myers Squibb, Hanall, Nicox, Kala, and Tarsus, although this work is unrelated to the current project. AD Pucker reports grants from Alcon, non-financial support from Art Optical, personal fees from CopperVision, Euclid Systems, Kala, and Nevakar, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures




References
-
- Bron AJ, Argueeso P, Irkec M, Bright FV. Clinical staining of the ocular surface: mechanisms and interpretations. Prog Retin Eye Res. 2015;44:36–61. - PubMed
-
- Feenstra RP, Tseng SC. Comparison of fluorescein and rose bengal staining. Ophthalmology. 1992;99(4):605–617. - PubMed
-
- Norn MS. Lissamine green. Vital staining of cornea and conjunctiva. Acta Ophthalmol. 1973;51(4):483–491. - PubMed
LinkOut - more resources
Full Text Sources

