The Effect of Longer Dosing Intervals for Long-Acting Injectable Antipsychotics on Outcomes in Schizophrenia
- PMID: 36915909
- PMCID: PMC10008005
- DOI: 10.2147/NDT.S395383
The Effect of Longer Dosing Intervals for Long-Acting Injectable Antipsychotics on Outcomes in Schizophrenia
Abstract
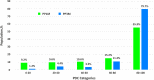
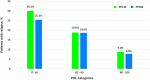
Medication nonadherence in schizophrenia can have serious implications including relapses and hospitalization. Long-acting injectable (LAI) antipsychotics require fewer administrations, while ensuring sustained medication coverage. In this review, we summarize the expected real-world benefits of longer dosing intervals in the management of schizophrenia. LAIs are associated with improved clinical outcomes of less frequent relapses and reduced functional impairment, encouraging patients to regain control of their lives. Aripiprazole lauroxil and paliperidone palmitate three-monthly (PP3M) LAIs have longer dosing intervals of 2-3 months and provide improved outcomes in patients with schizophrenia. Paliperidone palmitate six-monthly (PP6M) LAI provides the longest dosing interval, twice-yearly dosing, among existing LAIs. Decreasing the frequency of LAI administrations has the potential to reduce occurrence of serious outcomes associated with poor medication adherence. By eliminating the need for daily oral antipsychotic dosing, LAIs could increase the likelihood of patient acceptance, decrease stigma, and promote self-esteem. Longer intervals of medication coverage may be desirable for patients with higher risk of relapse including adults with recent-onset schizophrenia, those living in circumstances that may deprive them of regular access (eg, homeless), those that are in transitions between care settings or to reduce interpersonal contact during public health emergencies (eg, COVID-19 pandemic).
Keywords: adherence; long-acting injectable; paliperidone palmitate; schizophrenia.
© 2023 Milz et al.
Conflict of interest statement
John M. Kane has been a consultant for or received honoraria from Alkermes, Allergan, Boehringer-Ingelheim, Cerevel, Dainippon Sumitomo, HLS, Indivior, Intra-Cellular Therapies, Janssen, Johnson and Johnson, Karuna, LB Pharmaceuticals, Lundbeck, Lyndra, Merck, Minerva, Neurocrine Biosciences, Newron, Novartis, Otsuka, Pierre Fabre, Reviva, Roche, Saladex, Sunovion, Takeda, and Teva Pharmaceutical Industries; has received grant support from Otsuka, Lundbeck, Sunovion, and Janssen; and is a shareholder of LB Pharmaceuticals, North Shore Therapeutics, MedinCell, and Vanguard Research Group; royalties from Up to Date. Srihari Gopal was employed by Janssen Research & Development, LLC, USA when the study was conducted and is now employed by Regeneron Pharmaceuticals. All other authors are employees of Janssen and may hold company stocks or stock options. The authors report no other conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources