Therapeutic effect of α 7 nicotinic receptor activation after ischemic stroke in rats
- PMID: 36916034
- PMCID: PMC10369150
- DOI: 10.1177/0271678X231161207
Therapeutic effect of α 7 nicotinic receptor activation after ischemic stroke in rats
Abstract
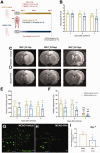
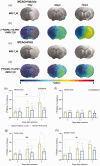
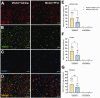
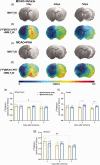
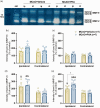
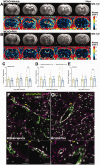
Nicotinic acetylcholine α7 receptors (α7 nAChRs) have a well-known modulator effect in neuroinflammation. Yet, the therapeutical effect of α7 nAChRs activation after stroke has been scarcely evaluated to date. The role of α7 nAChRs activation with PHA 568487 on inflammation after brain ischemia was assessed with positron emission tomography (PET) using [18F]DPA-714 and [18F]BR-351 radiotracers after transient middle cerebral artery occlusion (MCAO) in rats. The assessment of brain oedema, blood brain barrier (BBB) disruption and neurofunctional progression after treatment was evaluated with T2 weighted and dynamic contrast-enhanced magnetic resonance imaging (T2 W and DCE-MRI) and neurological evaluation. The activation of α7 nAChRs resulted in a decrease of ischemic lesion, midline displacement and cell neurodegeneration from days 3 to 7 after ischemia. Besides, the treatment with PHA 568487 improved the neurofunctional outcome. Treated ischemic rats showed a significant [18F]DPA-714-PET uptake reduction at day 7 together with a decrease of activated microglia/infiltrated macrophages. Likewise, the activation of α7 receptors displayed an increase of [18F]BR-351-PET signal in ischemic cortical regions, which resulted from the overactivation of MMP-2. Finally, the treatment with PHA 568487 showed a protective effect on BBB disruption and blood brain vessel integrity after cerebral ischemia.
Keywords: Cerebral ischemia; MRI; PET; metalloproteinases; neuroinflammation.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Gauberti M, Martinez de Lizarrondo S, Vivien D. Thrombolytic strategies for ischemic stroke in the thrombectomy era. J Thromb Haemost 2021; 19: 1618–1628. - PubMed
-
- Chamorro Á, Dirnagl U, Urra X, et al.. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol 2016; 15: 869–881. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

