Asymmetric catalysis by flavin-dependent halogenases
- PMID: 36916449
- PMCID: PMC11301518
- DOI: 10.1002/chir.23550
Asymmetric catalysis by flavin-dependent halogenases
Abstract
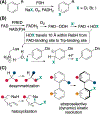
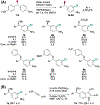
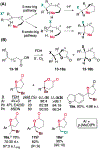
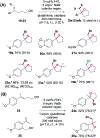
In nature, flavin-dependent halogenases (FDHs) catalyze site-selective chlorination and bromination of aromatic natural products. This ability has led to extensive efforts to engineer FDHs for selective chlorination, bromination, and iodination of electron rich aromatic compounds. On the other hand, FDHs are unique among halogenases and haloperoxidases that exhibit catalyst-controlled site selectivity in that no examples of enantioselective FDH catalysis in natural product biosynthesis have been characterized. Over the past several years, our group has established that FDHs can catalyze enantioselective reactions involving desymmetrization, atroposelective halogenation, and halocyclization. Achieving high activity and selectivity for these reactions has required extensive mutagenesis and mitigation of problems resulting from hypohalous acid generated during FDH catalysis. The single-component flavin reductase/FDH AetF is unique among the wild type enzyme we have studied in that it provides high activity and selectivity toward several asymmetric transformations. These results highlight the ability of FDH active sites to tolerate different substrate topologies and suggest that they could be useful for a broad range of oxidative halogenations.
Keywords: atroposelective; desymmetrization; halocyclization; halogenase; kinetic resolution.
© 2023 The Authors. Chirality published by Wiley Periodicals LLC.
Figures


References
-
- Naumann K Influence of chlorine substituents on biological activity of chemicals: a review. Pest Man Sci. 2000;56(1):3–21. doi:10.1002/(SICI)1526002D4998(200001)56:1003C3::AID002DPS107003E3.0.CO;2002DP - DOI
-
- de Meijere A, Bräse S, Oestreich M, Metal-catalyzed cross-coupling reactions and more, 2018.
-
- Gribble GW. A recent survey of naturally occurring organohalogen compounds. Environ Chem. 2015;12(4):396–405. doi:10.1071/EN15002 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

