Pembrolizumab plus chemotherapy in Japanese patients with triple-negative breast cancer: Results from KEYNOTE-355
- PMID: 36916728
- PMCID: PMC10225213
- DOI: 10.1002/cam4.5757
Pembrolizumab plus chemotherapy in Japanese patients with triple-negative breast cancer: Results from KEYNOTE-355
Abstract
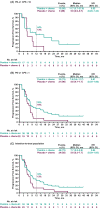
Pembrolizumab plus chemotherapy improved progression-free survival (PFS) and overall survival (OS) compared with placebo plus chemotherapy in patients with previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer with tumor programmed cell death ligand 1 (PD-L1) combined positive score (CPS) ≥10 in the global, phase 3, randomized controlled trial KEYNOTE-355. We report results for patients enrolled in Japan. Patients were randomized 2:1 to pembrolizumab 200 mg or placebo Q3W for 35 cycles plus chemotherapy (nab-paclitaxel, paclitaxel, or gemcitabine-carboplatin). Primary endpoints were PFS per RECIST version 1.1 by blinded independent central review and OS in patients with PD-L1 CPS ≥10, PD-L1 CPS ≥1, and the intention-to-treat (ITT) population. No alpha was assigned to this exploratory analysis. Eighty-seven patients were randomized in Japan (pembrolizumab plus chemotherapy, n = 61; placebo plus chemotherapy, n = 26), 66 (76%) had PD-L1 CPS ≥1, and 28 (32%) had PD-L1 CPS ≥10. Median time from randomization to data cutoff (June 15, 2021) was 44.7 (range, 37.2-52.9) months in the ITT population. Hazard ratios (HRs; 95% CI) for OS were 0.36 (0.14-0.89), 0.52 (0.30-0.91), and 0.46 (0.28-0.77) in the PD-L1 CPS ≥10, PD-L1 CPS ≥1, and ITT populations, respectively. HRs (95% CI) for PFS were 0.52 (0.20-1.34), 0.61 (0.35-1.06), and 0.64 (0.39-1.05). Grade 3 or 4 treatment-related adverse events occurred in 85% of patients in each group (no grade 5 events). Consistent with the global population, pembrolizumab plus chemotherapy tended to show improvements in OS and PFS with manageable toxicity versus placebo plus chemotherapy in Japanese patients and supports this combination in this setting.
Keywords: breast cancer; chemotherapy; clinical cancer research; clinical trials.
© 2023 Merck Sharp & Dohme LLC and The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Masaya Hattori has received honoraria from Eli Lilly and Daiichi Sankyo. Norikazu Masuda has provided leadership to Japan Breast Cancer Research Group Association (JBCRG); has received honoraria from AstraZeneca, Chugai Pharma, Eisai, Lilly Japan, and Pfizer; and has received research funding (all to institution) from AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Eli‐Lilly, Kyowa‐Kirin, MSD, Novartis, Pfizer, and Sanofi. Toshimi Takano has received honoraria from Daiichi‐Sankyo, Chugai, Eisai, Eli Lilly, and Celltrion and has received research funding (all to institution) from MSD, Daiichi‐Sankyo, Chugai, Eisai, and Ono. Koichiro Tsugawa has received manuscript fees from Pfizer and Eli Lilly; has received research funding from Konica Minolta, Inc; and has received scholarship endowments/research grants from Taiho Pharmaceutical Co., ltd and Chugai Pharmaceutical Co., ltd. Kenichi Inoue has received research funding (all to institution) from Astellas, AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eli‐Lilly, MSD, Novartis, Ono, Pfizer, Sanofi, Takeda, and Taiho. Koji Matsumoto has received honoraria from Kyowa‐Kirin and Chugai and has received research funding from MSD, Eli Lilly, Daiichi‐Sankyo, Chugai, Eisai, and ICON‐Japan. Takashi Ishikawa has received honoraria from Daiichi Sankyo, Kyowa Kirin, Pfizer, and Chugai. Mitsuya Itoh has no conflicts of interest. Hiroyuki Yasojima has no conflicts of interest. Yuko Tanabe has received research funding from MSD. Keiko Yamamoto is an employee of MSD K.K., Tokyo, Japan. Masato Suzuki is an employee of MSD K.K., Tokyo, Japan. Wilbur Pan is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and stockholder in Merck & Co., Inc., Rahway, NJ, USA. Javier Cortes has been a consultant/advisor for Roche, Celgene, Cellestia, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Merck Sharp & Dohme, GSK, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, Hibercell, BioInvent, Gemoab, Gilead, Menarini, Zymeworks, and Reveal Genomics; has received honoraria from Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, Merck Sharp & Dohme, and Daiichi Sankyo; has received research funding (all to institution) from Roche, Ariad pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer healthcare, Eisai, F.Hoffman‐La Roche, Guardant health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Puma C, and Queen Mary University of London; has stock in MedSIR, Nektar Pharmaceuticals, and Leuko (relative); and has received travel/accommodation expenses from Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo, Astrazeneca, and Gilead. In addition, Javier Cortes holds the following patents: (1) Pharmaceutical Combinations of A Pi3k Inhibitor And A Microtubule Destabilizing Agent. Javier Cortés Castán, Alejandro Piris Giménez, Violeta Serra Elizalde. WO 2014/199294 A and (2) Her2 as a predictor of response to dual HER2 blockade in the absence of cytotoxic therapy. Aleix Prat, Antonio Llombart, Javier Cortés. US 2019/ 0338368 A1. Hiroji Iwata has no conflicts of interest. The study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. All authors had access to the data from the study and had final responsibility for the decision to submit for publication.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Foundation for Promotion of Cancer Research . Cancer Statistics in Japan—2021. Accessed March 22, 2022. https://ganjoho.jp/public/qa_links/report/statistics/2021_en.html
-
- Yap YS, Lu YS, Tamura K, et al. Insights into breast cancer in the east vs the west: a review. JAMA Oncol. 2019;5(10):1489‐1496. - PubMed
-
- Ogiya R, Niikura N, Kumamaru H, et al. Breast cancer survival among Japanese individuals and US residents of Japanese and other origins: a comparative registry‐based study. Breast Cancer Res Treat. 2020;184(2):585‐596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

