vaRHC: an R package for semi-automation of variant classification in hereditary cancer genes according to ACMG/AMP and gene-specific ClinGen guidelines
- PMID: 36916756
- PMCID: PMC10032633
- DOI: 10.1093/bioinformatics/btad128
vaRHC: an R package for semi-automation of variant classification in hereditary cancer genes according to ACMG/AMP and gene-specific ClinGen guidelines
Abstract
Motivation: Germline variant classification allows accurate genetic diagnosis and risk assessment. However, it is a tedious iterative process integrating information from several sources and types of evidence. It should follow gene-specific (if available) or general updated international guidelines. Thus, it is the main burden of the incorporation of next-generation sequencing into the clinical setting.
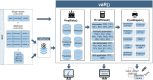
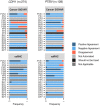
Results: We created the vaRiants in HC (vaRHC) R package to assist the process of variant classification in hereditary cancer by: (i) collecting information from diverse databases; (ii) assigning or denying different types of evidence according to updated American College of Molecular Genetics and Genomics/Association of Molecular Pathologist gene-specific criteria for ATM, CDH1, CHEK2, MLH1, MSH2, MSH6, PMS2, PTEN, and TP53 and general criteria for other genes; (iii) providing an automated classification of variants using a Bayesian metastructure and considering CanVIG-UK recommendations; and (iv) optionally printing the output to an .xlsx file. A validation using 659 classified variants demonstrated the robustness of vaRHC, presenting a better criteria assignment than Cancer SIGVAR, an available similar tool.
Availability and implementation: The source code can be consulted in the GitHub repository (https://github.com/emunte/vaRHC) Additionally, it will be submitted to CRAN soon.
© The Author(s) 2023. Published by Oxford University Press.
Figures


References
-
- Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57:289–300.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

