Molluscum Contagiosum Virus Protein MC008 Targets NF-κB Activation by Inhibiting Ubiquitination of NEMO
- PMID: 36916940
- PMCID: PMC10062130
- DOI: 10.1128/jvi.00108-23
Molluscum Contagiosum Virus Protein MC008 Targets NF-κB Activation by Inhibiting Ubiquitination of NEMO
Abstract
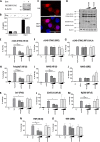
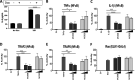
Molluscum contagiosum virus (MCV) is a human-adapted poxvirus that causes a common and persistent yet mild infection characterized by distinct, contagious, papular skin lesions. These lesions are notable for having little or no inflammation associated with them and can persist for long periods without an effective clearance response from the host. Like all poxviruses, MCV encodes potent immunosuppressive proteins that perturb innate immune pathways involved in virus sensing, the interferon response, and inflammation, which collectively orchestrate antiviral immunity and clearance, with several of these pathways converging at common signaling nodes. One such node is the regulator of canonical nuclear factor kappa B (NF-κB) activation, NF-κB essential modulator (NEMO). Here, we report that the MCV protein MC008 specifically inhibits NF-κB through its interaction with NEMO, disrupting its early ubiquitin-mediated activation and subsequent downstream signaling. MC008 is the third NEMO-targeting inhibitor to be described in MCV to date, with each inhibiting NEMO activation in distinct ways, highlighting strong selective pressure to evolve multiple ways of disabling this key signaling protein. IMPORTANCE Inflammation lies at the heart of most human diseases. Understanding the pathways that drive this response is the key to new anti-inflammatory therapies. Viruses evolve to target inflammation; thus, understanding how they do this reveals how inflammation is controlled and, potentially, how to disable it when it drives disease. Molluscum contagiosum virus (MCV) has specifically evolved to infect humans and displays an unprecedented ability to suppress inflammation in our tissue. We have identified a novel inhibitor of human innate signaling from MCV, MC008, which targets NEMO, a core regulator of proinflammatory signaling. Furthermore, MC008 appears to inhibit early ubiquitination, thus interrupting later events in NEMO activation, thereby validating current models of IκB kinase (IKK) complex regulation.
Keywords: MC008; NEMO; NF-κB; human; innate; innate immunity; molluscum contagiosum; ubiquitin; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

