Topical Ocular TRPV1 Antagonist SAF312 (Libvatrep) for Postoperative Pain After Photorefractive Keratectomy
- PMID: 36917119
- PMCID: PMC10020951
- DOI: 10.1167/tvst.12.3.7
Topical Ocular TRPV1 Antagonist SAF312 (Libvatrep) for Postoperative Pain After Photorefractive Keratectomy
Abstract
Purpose: Evaluation of safety and efficacy of topical ocular SAF312 (Libvatrep) in post-photorefractive keratectomy (PRK) pain.
Methods: In this placebo (vehicle)-controlled, participant- and investigator-masked study, 40 participants were randomized (1:1) to two treatment sequences in a bilateral PRK crossover design (SAF312 2.5% followed by vehicle [or vice versa], one eye drop, four times daily for 72 hours after PRK). Primary endpoints were visual analog scale (VAS) pain scores at 6 hours after first drop of study drug and average VAS scores over 0 to 12 hours postoperatively. Secondary endpoints included postoperative oral rescue medication (ORM) use and adverse events (AEs).
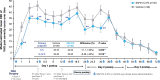
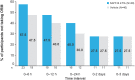
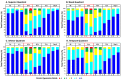
Results: All 40 participants completed the study. Both primary endpoints were met; mean difference in VAS pain scores between SAF312- and vehicle-treated eyes was -11.13 (P = 0.005, -25%) at 6 hours postoperatively and -8.56 (P = 0.017, -22%) over 0 to 12 hours. Mean VAS pain scores with SAF312 were consistently lower than with vehicle from 1 hour postoperatively up to 30 hours (P ≤ 0.10 observed in 8/11 time points). Less ORM was taken with SAF312 up to 0 to 72 hours postoperatively, with a trend of fewer participants taking ORM at 0 to 24 hours postoperatively with SAF312 versus vehicle. No serious AEs were reported. All ocular AEs were mild and transient, and none were drug related. SAF312-treated eyes showed no delay in wound healing and had a lower grade 4 conjunctival hyperemia 24 hours postoperatively versus vehicle-treated eyes.
Conclusions: SAF312 was well tolerated and effective in reducing ocular pain post-PRK.
Translational relevance: Topical SAF312 presents a new therapeutic option for patients undergoing PRK.
Conflict of interest statement
Disclosure:
Figures


References
-
- Müller LJ, Marfurt CF, Kruse F, Tervo TMT.. Corneal nerves: Structure, contents and function. Exp Eye Res. 2003; 76(5): 521–542. - PubMed
-
- Woreta FA, Gupta A, Hochstetler B, Bower KS.. Management of post-photorefractive keratectomy pain. Surv Ophthalmol . 2013; 58(6): 529–535. - PubMed
-
- Arshinoff SA, Mills MD, Haber S.. Pharmacotherapy of photorefractive keratectomy. J Cataract Refract Surg. 1996; 22(8): 1037–1044. - PubMed
-
- Faktorovich EG, Basbaum AI.. Effect of topical 0.5% morphine on postoperative pain after photorefractive keratectomy. J Refract Surg. 2010; 26(12): 934–941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

