Plasmin and plasminogen prevent sepsis severity by reducing neutrophil extracellular traps and systemic inflammation
- PMID: 36917195
- PMCID: PMC10243804
- DOI: 10.1172/jci.insight.166044
Plasmin and plasminogen prevent sepsis severity by reducing neutrophil extracellular traps and systemic inflammation
Abstract
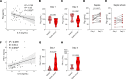
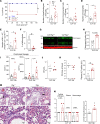
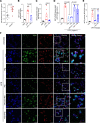
Sepsis is a lethal syndrome characterized by systemic inflammation and abnormal coagulation. Despite therapeutic advances, sepsis mortality remains substantially high. Herein, we investigated the role of the plasminogen/plasmin (Plg/Pla) system during sepsis. Plasma levels of Plg were significantly lower in mice subjected to severe compared with nonsevere sepsis, whereas systemic levels of IL-6, a marker of sepsis severity, were higher in severe sepsis. Plg levels correlated negatively with IL-6 in both septic mice and patients, whereas plasminogen activator inhibitor-1 levels correlated positively with IL-6. Plg deficiency render mice susceptible to nonsevere sepsis induced by cecal ligation and puncture (CLP), resulting in greater numbers of neutrophils and M1 macrophages, liver fibrin(ogen) deposition, lower efferocytosis, and increased IL-6 and neutrophil extracellular trap (NET) release associated with organ damage. Conversely, inflammatory features, fibrin(ogen), and organ damage were substantially reduced, and efferocytosis was increased by exogenous Pla given during CLP- and LPS-induced endotoxemia. Plg or Pla protected mice from sepsis-induced lethality and enhanced the protective effect of antibiotics. Mechanistically, Plg/Pla-afforded protection was associated with regulation of NET release, requiring Pla-protease activity and lysine binding sites. Plg/Pla are important host-protective players during sepsis, controlling local and systemic inflammation and collateral organ damage.
Keywords: Bacterial infections; Infectious disease; Plasmin.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

