Defibrillation effectiveness and safety of the shock waveform used in a contemporary wearable cardioverter defibrillator: Results from animal and human studies
- PMID: 36917566
- PMCID: PMC10013906
- DOI: 10.1371/journal.pone.0281340
Defibrillation effectiveness and safety of the shock waveform used in a contemporary wearable cardioverter defibrillator: Results from animal and human studies
Abstract
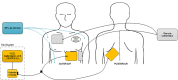
Introduction: The wearable cardioverter defibrillator (WCD) is used to protect patients at risk for sudden cardiac arrest. We examined defibrillation efficacy and safety of a biphasic truncated exponential waveform designed for use in a contemporary WCD in three animal studies and a human study.
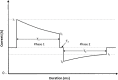
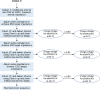
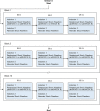
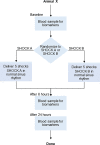
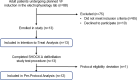
Methods: Animal (swine) studies: #1: Efficacy comparison of a 170J BTE waveform (SHOCK A) to a 150J BTE waveform (SHOCK B) that approximates another commercially available waveform. Primary endpoint first shock success rate. #2: Efficacy comparison of the two waveforms at attenuated charge voltages in swine at three prespecified impedances. Primary endpoint first shock success rate. #3: Safety comparison of SHOCK A and SHOCK B in swine. Primary endpoint cardiac biomarker level changes baseline to 6 and 24 hours post-shock. Human Study: Efficacy comparison of SHOCK A to prespecified goal and safety evaluation. Primary endpoint cumulative first and second shock success rate. Safety endpoint adverse events.
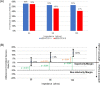
Results: Animal Studies #1: 120 VF episodes in six swine. First shock success rates for SHOCK A and SHOCK B were 100%; SHOCK A non-inferior to SHOCK B (entire 95% CI of rate difference above -10% margin, p < .001). #2: 2,160 VF episodes in thirty-six swine. Attenuated SHOCK A was non-inferior to attenuated SHOCK B at each impedance (entire 95% CI of rate difference above -10% margin, p < .001). #3: Ten swine, five shocked five times each with SHOCK A, five shocked five times each with SHOCK B. No significant difference in troponin I (p = 0.658) or creatine phosphokinase (p = 0.855) changes from baseline between SHOCK A and SHOCK B. Human Study: Thirteen patients, 100% VF conversion rate. Mild skin irritation from adhesive defibrillation pads in three patients.
Conclusions: The BTE waveform effectively and safely terminated induced VF in swine and a small sample in humans.
Trial registration: Human study clinical trial registration: URL: https://clinicaltrials.gov; Unique identifier: NCT04132466.
Copyright: © 2023 Gleva et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: One US patent application related to the research presented in this manuscript - Defibrillation Waveforms for a Wearable Cardiac Defibrillator (Pub. No. US 2021/0196965 A1; Pub. Date: Jul 1, 2021) Taylor TG and Medema DK; “ASSURE WCD System” is a product of Kestra Medical Technologies, Inc. There are no other patents, products in development or marketed products to declare. Joseph Sullivan, Kelly Brennan, Ron K. Rowbotham and Laura M. Gustavson are employees and stockholders of Kestra Medical Technologies, Inc.; Marye J. Gleva reports modest speaking honoraria from ZOLL Medical and Gaffney Events Educational Trust, modest consulting honoraria from Kestra Medical Technologies, Inc., compensation from the University of Rochester (Rochester NY) and Prairie Education and Research Institute (Springfield, IL) for research committee participation; Greg Walcott reports institutional research support from Kestra Medical Technologies, Inc.; Kelley R. Branch reports institutional research grant support from Kestra Medical Technologies, Inc.; Jeanne E. Poole reports institutional research grant support from Kestra Medical Technologies, Inc., Biotronik and Atricure, compensation from the University of Rochester (Rochester NY) for research committee participation and from the Heart Rhythm Society for Core Concepts Educational Course and for Editor-in-Chief for the Heart Rhythm O2Journal. Jeanne Poole also reports honoraria from Medtronic for service on the Executive Committee for the WRAP_IT study and from Boston Scientific for participation on the Medical Advisory Board. The remaining authors declare no conflict of interest. This support does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Panchal AR, Bartos JA, JG, Donnino MW, Drennan IR, Karen G. Hirsch KG, et al.. On behalf of the Adult Basic and Advanced Life Support Writing Group. Part 3: Adult Basic and Advanced Life Support 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(suppl 2): S366–S468. doi: 10.1161/CIR.000000000000091 - DOI - PubMed
-
- Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 6: Advanced Cardiovascular Life Support. Section 2: Defibrillation. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000. Aug 22;102(8 Suppl): I90–4. doi: 10.1161/circ.102.suppl_1.I-90 - DOI - PubMed
-
- Higgins SL, Herre JM, Epstein AE, Greer GS, Friedman PL, Gleva ML et al.. A Comparison of Biphasic and Monophasic Shocks for External Defibrillation. Prehospital Emergency Care. 2000;4: 305–313. doi: 10.1080/10903120090941001 and “Erratum.” Prehospital Emergency Care. 2001; 5:78. 10.1080/10903120190940380 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

