Immune Profiling Panel Gene Set Identifies Critically Ill Patients With Low Monocyte Human Leukocyte Antigen-DR Expression: Preliminary Results From the REAnimation Low Immune Status Marker (REALISM) Study
- PMID: 36917594
- PMCID: PMC10187625
- DOI: 10.1097/CCM.0000000000005832
Immune Profiling Panel Gene Set Identifies Critically Ill Patients With Low Monocyte Human Leukocyte Antigen-DR Expression: Preliminary Results From the REAnimation Low Immune Status Marker (REALISM) Study
Abstract
Objectives: There is a crucial unmet need for biomarker-guided diagnostic and prognostic enrichment in clinical trials evaluating immune modulating therapies in critically ill patients. Low monocyte expression of human leukocyte antigen-DR (mHLA-DR), considered as a reference surrogate to identify immunosuppressed patients, has been proposed for patient stratification in immunostimulation approaches. However, its widespread use in clinic has been somewhat hampered by technical constraints inherent to flow cytometry technology. The objective of the present study was to evaluate the ability of a prototype multiplex polymerase chain reaction tool (immune profiling panel [IPP]) to identify immunosuppressed ICU patients characterized by a low mHLA-DR expression.
Design: Retrospective observational cohort study.
Setting: Adult ICU in a University Hospital, Lyon, France.
Patients: Critically ill patients with various etiologies enrolled in the REAnimation Low Immune Status Marker study (NCT02638779).
Interventions: None.
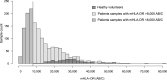
Measurements and main results: mHLA-DR and IPP data were obtained from 1,731 blood samples collected from critically ill patients with various etiologies and healthy volunteers. A partial least square regression model combining the expression levels of IPP markers was trained and used for the identification of samples from patients presenting with evidence of immunosuppression, defined here as mHLADR less than 8,000 antibodies bound per cell (AB/C). The IPP gene set had an area under the receiver operating characteristic curve (AUC) of 0.86 (95% CI 0.83-0.89) for the identification of immunosuppressed patients. In addition, when applied to the 123 patients still in the ICU at days 5-7 after admission, IPP similarly enriched the number of patients with ICU-acquired infections in the immunosuppressed group (26%), in comparison with low mHLA-DR (22%).
Conclusions: This study reports on the potential of the IPP gene set to identify ICU patients presenting with mHLA-DR less than 8,000 AB/C. Upon further optimization and validation, this molecular tool may help in the stratification of patients that could benefit from immunostimulation in the context of personalized medicine.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and Wolters Kluwer Health, Inc.
Conflict of interest statement
Drs. Peronnet, Blein, Cerrato, Fleurie, Llitjos, Textoris, and Brengel-Pesce are employees of bioMérieux. Drs. Peronnet, Cerrato, Fleurie, Llitjos, Kreitmann, Terraz, Conti, Rimmelé, Lukaszewicz, Brengel-Pesce, and Monneret work in a joint research unit, cofunded by the Hospices Civils de Lyon and bioMérieux. Drs. Peronnet, Venet, and Monneret are coinventors in patent applications covering the following markers: CX3CR1 and S100A9 . Drs. Peronnet, Venet, Rimmelé, Textoris, and Monneret are coinventors in patent applications covering the following markers: CX3CR1, IL1R2, C3AR1, CD177, CIITA, and TAP2 . BioFire—a bioMérieux company—holds patents on the technology. This does not alter the authors’ adherence to all the policies on sharing data and materials. Drs. Peronnet’s, Blein’s, Cerrato’s, Fleurie’s, Llitjos’, Terraz’s, and Lukaszewicz’s institutions received funding from the Agence Nationale de la Recherche; they received support for article research from bioMérieux, Sanofi, and GlaxoSmithKline. Drs. Peronnet, Blein, Cerrato, Fleurie, Kreitmann, Terraz, Textoris, and Brengel-Pesce received funding from bioMérieux. Drs. Peronnet, Cerrato, and Lukaszewicz disclosed that they are coinventors on patent applications. Dr. Peronnet disclosed that her partner is employed by bioMérieux. The remaining authors have disclosed that they do not have any potential conflict of interest.
Figures

References
-
- Marshall JC: Why have clinical trials in sepsis failed? Trends Mol Med 2014; 20:195–203 - PubMed
-
- Cavaillon JM, Annane D: Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res 2006; 12:151–170 - PubMed
-
- Torres LK, Pickkers P, van der Poll T: Sepsis-induced immunosuppression. Annu Rev Physiol 2022; 84:157–181 - PubMed
-
- Venet F, Textoris J, Blein S, et al. : Immune profiling demonstrates a common immune signature of delayed acquired immunodeficiency in patients with various etiologies of severe injury. Crit Care Med 2021; 50:565–575 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

