Dysregulation of ribosome-associated quality control elicits cognitive disorders via overaccumulation of TTC3
- PMID: 36917672
- PMCID: PMC10041068
- DOI: 10.1073/pnas.2211522120
Dysregulation of ribosome-associated quality control elicits cognitive disorders via overaccumulation of TTC3
Abstract
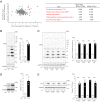
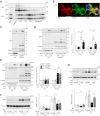
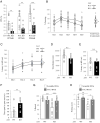
Ribosome-associated quality control (RQC) pathway is responsible for degradation of nascent polypeptides in aberrantly stalled ribosomes, and its defects may lead to neurological diseases. However, the underlying molecular mechanism of how RQC dysfunction elicits neurological disorders remains poorly understood. Here we revealed that neurons with knockout (KO) of ubiquitin ligase LTN1, a key gene in the RQC pathway, show developmental defects in neurons via upregulation of TTC3 and UFMylation signaling proteins. The abnormally enhanced TTC3 protein in Ltn1 KO neurons reduced further accumulation of translationally arrested products by preventing translation initiation of selective genes. However, the overaccumulated TTC3 protein in turn caused dendritic abnormalities and reduced surface-localized GABAA receptors during neuronal development. Ltn1 KO mice showed behavioral deficits associated with cognitive disorders, a subset of which were restored by TTC3 knockdown in medial prefrontal cortex. Together, the overactivated cellular compensatory mechanism against defective RQC through TTC3 overaccumulation induced synaptic and cognitive deficits. More broadly, these findings represent a novel cellular mechanism underlying neuronal dysfunctions triggered by exaggerated cellular stress response to accumulated abnormal translation products in neurons.
Keywords: TTC3; UFMylation; cognitive disorders; ribosome stalling; ribosome-associated quality control.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Hartl F. U., Protein misfolding diseases. Annu. Rev. Biochem. 86, 21–26 (2017). - PubMed
-
- Hipp M. S., Kasturi R., Hartl F. U., The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 20, 421–435 (2017). - PubMed
-
- Balchin D., Hayer-Hartl M., Hartl F. U., In vivo aspects of protein folding and quality control. Science 353, aac4354 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

