Two-photon fluorescence imaging and specifically biosensing of norepinephrine on a 100-ms timescale
- PMID: 36918539
- PMCID: PMC10014876
- DOI: 10.1038/s41467-023-36869-3
Two-photon fluorescence imaging and specifically biosensing of norepinephrine on a 100-ms timescale
Abstract
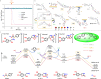
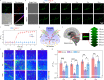
Norepinephrine (NE) is a key neurotransmitter in the central nervous system of organisms; however, specifically tracking the transient NE dynamics with high spatiotemporal resolution in living systems remains a great challenge. Herein, we develop a small molecular fluorescent probe that can precisely anchor on neuronal cytomembranes and specifically respond to NE on a 100-ms timescale. A unique dual acceleration mechanism of molecular-folding and water-bridging is disclosed, which boosts the reaction kinetics by ˃105 and ˃103 times, respectively. Benefiting from its excellent spatiotemporal resolution, the probe is applied to monitor NE dynamics at the single-neuron level, thereby, successfully snapshotting the fast fluctuation of NE levels at neuronal cytomembranes within 2 s. Moreover, two-photon fluorescence imaging of acute brain tissue slices reveals a close correlation between downregulated NE levels and Alzheimer's disease pathology as well as antioxidant therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

