Association of SARS-CoV-2 BA.4/BA.5 Omicron lineages with immune escape and clinical outcome
- PMID: 36918548
- PMCID: PMC10012300
- DOI: 10.1038/s41467-023-37051-5
Association of SARS-CoV-2 BA.4/BA.5 Omicron lineages with immune escape and clinical outcome
Abstract
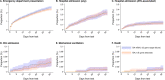
Expansion of the SARS-CoV-2 BA.4 and BA.5 Omicron subvariants in populations with prevalent immunity from prior infection and vaccination, and associated burden of severe COVID-19, has raised concerns about epidemiologic characteristics of these lineages including their association with immune escape or severe clinical outcomes. Here we show that BA.4/BA.5 cases in a large US healthcare system had at least 55% (95% confidence interval: 43-69%) higher adjusted odds of prior documented infection than time-matched BA.2 cases, as well as 15% (9-21%) and 38% (27-49%) higher adjusted odds of having received 3 and ≥4 COVID-19 vaccine doses, respectively. However, after adjusting for differences in epidemiologic characteristics among cases with each lineage, BA.4/BA.5 infection was not associated with differential risk of emergency department presentation, hospital admission, or intensive care unit admission following an initial outpatient diagnosis. This finding held in sensitivity analyses correcting for potential exposure misclassification resulting from unascertained prior infections. Our results demonstrate that the reduced severity associated with prior (BA.1 and BA.2) Omicron lineages, relative to the Delta variant, has persisted with BA.4/BA.5, despite the association of BA.4/BA.5 with increased risk of breakthrough infection among previously vaccinated or infected individuals.
© 2023. The Author(s).
Conflict of interest statement
J.A.L. has received research grants and consulting honoraria unrelated to this study from Pfizer. S.Y.T. has received research grants unrelated to this study from Pfizer. The remaining authors disclose no competing interests.
Figures

References
-
- US Centers for Disease Control and Prevention. COVID Data Tracker. Accessed 2 November, 2022. Available from: https://covid.cdc.gov/covid-data-tracker.
-
- Tartof SY, et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case–control study. Lancet Respir. Med. 2022;10:689–699. doi: 10.1016/S2213-2600(22)00101-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

