Analytical and clinical validity of wearable, multi-sensor technology for assessment of motor function in patients with Parkinson's disease in Japan
- PMID: 36918552
- PMCID: PMC10015076
- DOI: 10.1038/s41598-023-29382-6
Analytical and clinical validity of wearable, multi-sensor technology for assessment of motor function in patients with Parkinson's disease in Japan
Abstract
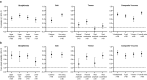
Continuous, objective monitoring of motor signs and symptoms may help improve tracking of disease progression and treatment response in Parkinson's disease (PD). This study assessed the analytical and clinical validity of multi-sensor smartwatch measurements in hospitalized and home-based settings (96 patients with PD; mean wear time 19 h/day) using a twice-daily virtual motor examination (VME) at times representing medication OFF/ON states. Digital measurement performance was better during inpatient clinical assessments for composite V-scores than single-sensor-derived features for bradykinesia (Spearman |r|= 0.63, reliability = 0.72), tremor (|r|= 0.41, reliability = 0.65), and overall motor features (|r|= 0.70, reliability = 0.67). Composite levodopa effect sizes during hospitalization were 0.51-1.44 for clinical assessments and 0.56-1.37 for VMEs. Reliability of digital measurements during home-based VMEs was 0.62-0.80 for scores derived from weekly averages and 0.24-0.66 for daily measurements. These results show that unsupervised digital measurements of motor features with wrist-worn sensors are sensitive to medication state and are reliable in naturalistic settings.Trial Registration: Japan Pharmaceutical Information Center Clinical Trials Information (JAPIC-CTI): JapicCTI-194825; Registered June 25, 2019.
© 2023. The Author(s).
Conflict of interest statement
T.H. has received grants from: the Japan Agency for Medical Research and Development (grant numbers: 20dm0107156, 21wm0425015, 21ak0101112, and 21dk0207055), the Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research (grant number: 21K07424), the Setsuro Fujii Memorial Osaka Foundation for Promotion of Fundamental Medical Research, and Daiichi Sankyo TaNeDS; and research funds from: Daiichi Sankyo TaNeDS Funding Program. He has received speaker’s honoraria from: Sumitomo Dainippon Pharma Co. Ltd., Takeda Pharmaceutical Company Limited, FP Pharmaceutical Corporation, Kyowa Kirin Co., Ltd., Nihon Medi-Physics Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd, and Otsuka Pharmaceutical Co., Ltd. N.N. has received grants from Grant-in-Aid for Scientific Research (Kakenhi) and Sumitomo Dainippon Pharma Co. Ltd. and honoraria from Sumitomo Dainippon Pharma Co. Ltd., Takeda Pharmaceutical Company Limited, AbbVie GK, Kyowa Kirin Co., Ltd., and Ono Pharmaceutical Co., Ltd. N.H. has received consulting fees from GlaxoSmithKline K.K., AbbVie Inc., Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co. Ltd., Kyowa Kirin Co., Ltd., Hisamitsu Pharmaceutical Co., Inc., Meiji Seika Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd, and FP Pharmaceutical Corporation; lecture fees from MSD K.K., Eli Lilly Japan K.K., Eisai Co., Ltd., FP Pharmaceutical Corporation, Otsuka Pharmaceutical Co., Ltd., Tsumura & Co., Kyowa Kirin Co., Ltd., GlaxoSmithKline K.K., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Nihon Medi-Physics Co., Ltd., Novartis Pharma K.K., Pfizer Japan Inc., Nippon Boehringer Ingelheim Co., Ltd., Sumitomo Dainippon Pharma Co. Ltd., and Daiichi Sankyo Company, Limited; honoraria from FP Pharmaceutical Corporation, Novartis Pharma K.K., Kyowa Kirin Co., Ltd., and AbbVie Inc.; research support from Otsuka Pharmaceutical Co., Ltd.; and grants from Astellas Pharma Inc., Eisai Co., Ltd., GlaxoSmithKline K.K., Sumitomo Dainippon Pharma Co. Ltd., Takeda Pharmaceutical Company Limited, Novartis Pharma K.K., Pfizer Japan Inc., Kyowa Kirin Co., Ltd., Medtronic Japan Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Boston Scientific Corporation, Kissei Pharmaceutical Co., Ltd, and Otsuka Pharmaceutical Co., Ltd. G.O. has received grants from Grant-in-Aid for Scientific Research (Kakenhi) and Novartis Pharma K.K. and honoraria from AbbVie Inc., Boston Scientific Corporation, Eisai Co., Ltd., Kyowa Kirin Co., Ltd., Medtronic Japan Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co. Ltd., and Takeda Pharmaceutical Company Limited. A.Hat, M.I., H.K., R.N., T.O., Y.O., F.S., K.S., H.T.-A., D.T., S.S., and S.U. have no conflicts of interest to disclose. M.B., C.C., K.C.S., R.K., W.J.M., and E.R. are employees and minor shareholders of Verily Life Sciences LLC. K.F., Y.F., A.Hay, M.M., and K.N. are employees of Takeda Pharmaceutical Company Limited. J.F. was an employee of Takeda at the time the work was conducted and is a minor shareholder of Takeda and GlaxoSmithKline.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

