Sensory evaluation of the bitterness of asenapine using D-sorbitol pretreatment: single-blind, placebo-controlled, crossover trial
- PMID: 36918838
- PMCID: PMC10012564
- DOI: 10.1186/s12888-023-04664-5
Sensory evaluation of the bitterness of asenapine using D-sorbitol pretreatment: single-blind, placebo-controlled, crossover trial
Abstract
Background: Antipsychotics are essential in the acute treatment of and maintenance therapy for schizophrenia, but medication adherence and long-term treatment continuity are needed to maximize their effectiveness. Each antipsychotic has various side effects, which may affect adherence. Some patients with schizophrenia are reluctant to take asenapine because of its unique oral-related side effects, such as the bitter taste caused by sublingual administration. Our previous basic research found that D-sorbitol lowered the bitterness parameters of the taste sensors. However, whether D-sorbitol has the same effect in patients remains unclear. Therefore, using a D-sorbitol solution, we aim to evaluate changes in the bitterness of asenapine among patients with schizophrenia.
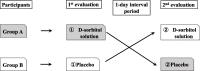
Methods: In this single-blind, placebo-controlled, crossover trial, we plan to recruit 20 adult patients with schizophrenia spectrum disorder who take sublingual asenapine tablets. The participants will be divided into two groups (n = 10 each). Each group will be given a D-sorbitol or placebo solution on the first day for rinsing before taking the sublingual asenapine tablets. After a 1-day interval, the participants will rinse their mouths again with a different liquid. Questionnaires regarding changes in taste and the willingness to continue asenapine will be conducted before the start of the study and after each rinse. The primary and secondary end points will be a taste evaluation of bitterness, and the willingness to continue asenapine, respectively. Differences in questionnaire scores between the D-sorbitol and placebo solutions will be calculated and analyzed using a McNemar test.
Discussion: This study aims to determine the efficacy of D-sorbitol in masking the bitter taste of asenapine. To our knowledge, it is the first intervention study using D-sorbitol for bitter taste of asenapine in patients with schizophrenia. Evidence of the efficacy of D-sorbitol could result in D-sorbitol pretreatment being an easy and inexpensive means of improving adherence to asenapine.
Trial registration: This study was registered in the Japan Registry of Clinical Trials jRCTs041210019, on May 14, 2021. Ethics approval was obtained from the Nagoya University Clinical Research Review Board.
Keywords: Adherence; Asenapine; Bitter taste of asenapine; D-Sorbitol pretreatment; Maintenance therapy; Schizophrenia; Side effects; Taste evaluation.
© 2023. The Author(s).
Conflict of interest statement
SW has no conflicts of interest to declare. KI has received speakers’ honoraria from Eisai, Kyowa, Meiji Seika Pharma, MSD, Otsuka, Sumitomo Dainippon, Taisho, Takeda, Towa, and Viatris. HO has no conflicts of interest to declare. HH has no conflicts of interest to declare. SH and AK are full-time employees of Meiji Seika Pharma Co., Ltd. DM has no conflicts of interest to declare. KY has received research support or speakers’ honoraria from Sumitomo Dainippon, Otsuka, Eisai, and DAIICHI SANKYO. NO has received research support or speakers’ honoraria from, or has served as a consultant to, Sumitomo Dainippon, Eisai, Otsuka, KAITEKI, Mitsubishi Tanabe, Shionogi, Eli Lilly, Mochida, DAIICHI SANKYO, Nihon Medi-Physics, Takeda, Meiji Seika Pharma, EA Pharma, Pfizer, MSD, Lundbeck Japan, Taisho Pharma, Janssen, UCB, Shionogi, Nihon Medi-Physics, Tsumura, Novartis, and Astellas.
Figures
References
-
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, Arndt T, Bäckers L, Rothe P, Cipriani A, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. doi: 10.1016/S0140-6736(19)31135-3. - DOI - PMC - PubMed
-
- Greger J, Aladeen T, Lewandowski E, Wojcik R, Westphal E, Rainka M, Capote H. Comparison of the Metabolic Characteristics of Newer Second Generation Antipsychotics: Brexpiprazole, Lurasidone, Asenapine, Cariprazine, and Iloperidone With Olanzapine as a Comparator. J Clin Psychopharmacol. 2021;41(1):5–12. doi: 10.1097/JCP.0000000000001318. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources