PI3k Inhibitors in NHL and CLL: An Unfulfilled Promise
- PMID: 36919100
- PMCID: PMC10008402
- DOI: 10.2147/BLCTT.S309171
PI3k Inhibitors in NHL and CLL: An Unfulfilled Promise
Abstract
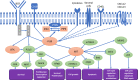
Phosphatidylinositol 3-kinases (PI3Ks) are a family of intracellular signal transducer enzymes that can attach a phosphate group to the 3'-hydroxyl of the inositol moiety of membrane-embedded phosphatidylinositol (PI). PI3Ks have been shown to play important roles in cell proliferation, growth, survival, motility, and metabolism. Nonetheless, the PI3K pathway has also shown to be overactivated in several tumors, particularly B-cell malignancies. In recent years, the PI3K signaling pathway has become the major focus of substantial drug discovery and development efforts. Selective (PI3K) inhibitors have been approved for the treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), and indolent non-Hodgkin lymphomas (iNHL), such as follicular lymphoma and marginal-zone lymphoma. Four selective PI3K inhibitors have received accelerated FDA approvals for the treatment of patients with relapsed/refractory (R/R) CLL and/or iNHL based mainly on single-arm Phase II studies: Idelalisib (PI3K-δ inhibitor), copanlisib (dual PI3K-α and PI3K-δ inhibitor), duvelisib (dual PI3K-γ and PI3K-δ inhibitor), and umbralisib (dual PI3Kδ and CK1ε inhibitor). Conversely, recent interim results of randomized control trials (RCTs) involving some of these agents, showed a worrisome trend of decrease in overall survival (OS), and an increase in fatal and severe adverse effects, in comparison with patients in the control arms. Consequently, the class of PI3K inhibitors came under scrutiny, with an FDA expert panel voting on April 21, 2022, recommending that future FDA approvals of PI3K inhibitors be supported by randomized data, rather than single-arm data only, and further discontinuing the use of almost all the PI3K inhibitors in hematologic malignancies. As we believe further research is needed to help potentialize PI3K inhibitors by improving their safety profiles, this mini-review aims at revisiting the clinical successes, the failures, and the promising aspect of this class of drugs, while presenting possible ways that could benefit its successful development.
Keywords: accelerated approval; clinical trials; hematologic malignancies; phosphatidylinositol-3 kinases; safety profile.
© 2023 Bou Zeid and Yazbeck.
Conflict of interest statement
Dr Victor Yazbeck reports grants, personal fees from Seagen, grants, personal fees from AstraZeneca, personal fees from ADC Therapeutics, personal fees from Verastem, grants, personal fees from Gilead, personal fees from TG Therapeutics, grants, personal fees from Beigene, grants, personal fees from Genmab, personal fees from MorphoSys, outside the submitted work. The authors report no conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous