Comparison of the Cost and Effect of Combined Conditioned Medium and Conventional Medium for Fallopian Tube Organoid Cultures
- PMID: 36919683
- PMCID: PMC10021093
- DOI: 10.1177/09636897231160216
Comparison of the Cost and Effect of Combined Conditioned Medium and Conventional Medium for Fallopian Tube Organoid Cultures
Abstract
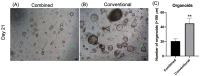
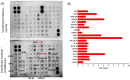
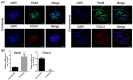
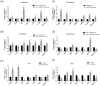
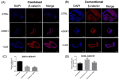
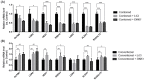
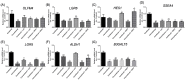
Fallopian tube epithelial cells (FTEC) are thought to be the cell of origin of high-grade serous ovarian carcinoma. FTEC organoids can be used as research models for the disease. Nevertheless, culturing organoids requires a medium supplemented with several expensive growth factors. We proposed that a combined conditioned medium based on the composition of the fallopian tubes, including epithelial, stromal, and endothelial cells could enhance FTEC organoid formation. We derived two primary culture cell lines from the fimbria portion of the fallopian tubes. The organoids were split into conventional or combined medium groups based on what medium they were grown in and compared. The number and size of the organoids were evaluated. Quantitative polymerase chain reaction (qPCR) and immunohistochemistry (IHC) were used to evaluate gene and protein expression (PAX8, FOXJ1, beta-catenin, and stemness genes). Enzyme-linked immunosorbent assay was used to measure Wnt3a and RSPO1 in both mediums. DKK1 and LiCl were added to the mediums to evaluate their influence on beta-catenin signaling. The growth factor in the combined medium was evaluated by the growth factor array. We found that the conventional medium was better for organoids regarding proliferation (number and size). In addition, WNT3A and RSPO1 concentrations were too low in the combined medium and needed to be added making the cost equivalent to the conventional medium. However, the organoid formation rate was 100% in both groups. Furthermore, the combined medium group had higher PAX8 and stemness gene expression (OLFM4, SSEA4, LGR5, B3GALT5) when compared with the conventional medium group. Wnt signaling was evident in the organoids grown in the conventional medium but not in the combined medium. PLGF, IGFBP6, VEGF, bFGF, and SCFR were found to be enriched in the combined medium. In conclusion, the combined medium could successfully culture organoids and enhance PAX8 and stemness gene expression. However, the conventional medium was a better medium for organoid proliferation. The expense of both mediums was comparable. The benefit of using a combined medium requires further exploration.
Keywords: Wnt/beta-catenin; endothelial cells; fallopian tube epithelial cells; medium; organoid; stromal cells.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Perrone MG, Luisi O, De Grassi A, Ferorelli S, Cormio G, Scilimati A. Translational theragnosis of ovarian cancer: where do we stand? Curr Med Chem. 2020;27(34):5675–715. - PubMed
-
- Wu NY, Fang C, Huang HS, Wang J, Chu TY. Natural history of ovarian high-grade serous carcinoma from time effects of ovulation inhibition and progesterone clearance of p53-defective lesions. Mod Pathol. 2020;33(1):29–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

