HMGA1 induces FGF19 to drive pancreatic carcinogenesis and stroma formation
- PMID: 36919699
- PMCID: PMC10014113
- DOI: 10.1172/JCI151601
HMGA1 induces FGF19 to drive pancreatic carcinogenesis and stroma formation
Abstract
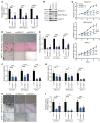
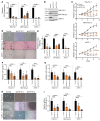
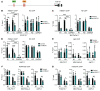
High mobility group A1 (HMGA1) chromatin regulators are upregulated in diverse tumors where they portend adverse outcomes, although how they function in cancer remains unclear. Pancreatic ductal adenocarcinomas (PDACs) are highly lethal tumors characterized by dense desmoplastic stroma composed predominantly of cancer-associated fibroblasts and fibrotic tissue. Here, we uncover an epigenetic program whereby HMGA1 upregulates FGF19 during tumor progression and stroma formation. HMGA1 deficiency disrupts oncogenic properties in vitro while impairing tumor inception and progression in KPC mice and subcutaneous or orthotopic models of PDAC. RNA sequencing revealed HMGA1 transcriptional networks governing proliferation and tumor-stroma interactions, including the FGF19 gene. HMGA1 directly induces FGF19 expression and increases its protein secretion by recruiting active histone marks (H3K4me3, H3K27Ac). Surprisingly, disrupting FGF19 via gene silencing or the FGFR4 inhibitor BLU9931 recapitulates most phenotypes observed with HMGA1 deficiency, decreasing tumor growth and formation of a desmoplastic stroma in mouse models of PDAC. In human PDAC, overexpression of HMGA1 and FGF19 defines a subset of tumors with extremely poor outcomes. Our results reveal what we believe is a new paradigm whereby HMGA1 and FGF19 drive tumor progression and stroma formation, thus illuminating FGF19 as a rational therapeutic target for a molecularly defined PDAC subtype.
Keywords: Cancer; Growth factors; Oncogenes; Oncology.
Figures







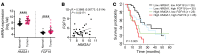

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

