Metabolic shift towards oxidative phosphorylation reduces cell-density-induced cancer-stem-cell-like characteristics in prostate cancer in vitro
- PMID: 36919762
- PMCID: PMC10110405
- DOI: 10.1242/bio.059615
Metabolic shift towards oxidative phosphorylation reduces cell-density-induced cancer-stem-cell-like characteristics in prostate cancer in vitro
Abstract
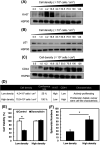
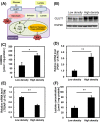
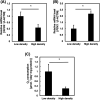
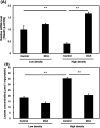
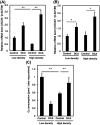
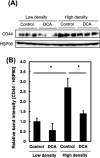
Numerous cancer patients undergoing conventional cancer therapies such as radiotherapy, chemotherapy and surgical tumour removal face relapses several years or even decades later. This may be due to the presence of cancer stem cells (CSCs) that survived said therapies. In this study, we aimed to uncover the relationship between cell density and CSCs, and the role of the Warburg effect in regulating CSC-like characteristics. A prostate cancer cell line, PC3, was used in this study. To investigate the Warburg effect effect and CSC-like characteristics in prostate cancer, we measured the expression levels of glycolysis and OXPHOS-related genes, and performed spheroid forming, cell viability and various glycolysis and OXPHOS-assays. We observed that increased cell density caused a metabolic shift from glycolysis to OXPHOS and higher CSC-like characteristics. However, the use of dichloroacetate (DCA), an inhibitor of the Warburg effect, significantly inhibited the cell-density-induced metabolic shift and CSC-like characteristics. Changes in cell density strongly influenced the preferred metabolic pathway of prostate cancer cells, regulating their CSC-like characteristics. It is possible that DCA, an inhibitor of the Warburg effect, could be a novel drug used to treat CSCs by distinguishing Warburg effect, preventing future cancer relapses.
Keywords: Cancer stem cell; Glycolysis; Oxidative phosphorylation; Prostate cancer; Warburg effect.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Bahmad, H. F., Cheaito, K., Chalhoub, R. M., Hadadeh, O., Monzer, A., Ballout, F., El-Hajj, A., Mukherji, D., Liu, Y. N., Daoud, G.et al. (2018). Sphere-formation assay: three-dimensional in vitro culturing of prostate cancer stem/progenitor sphere-forming cells. Front. Oncol. 8, 347. 10.3389/fonc.2018.00347 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

