Efficacy and safety of golimumab in patients with non-radiographic axial spondyloarthritis: a withdrawal and retreatment study (GO-BACK)
- PMID: 36919768
- PMCID: PMC10629786
- DOI: 10.1093/rheumatology/kead112
Efficacy and safety of golimumab in patients with non-radiographic axial spondyloarthritis: a withdrawal and retreatment study (GO-BACK)
Abstract
Objectives: The GO-BACK study was designed to evaluate the efficacy and safety of golimumab (GLM) treatment withdrawal in adults with non-radiographic axial spondyloarthritis (nr-axSpA) who demonstrate inactive disease during a 10-month open-label (OL) GLM run-in.
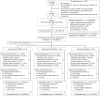
Methods: Eligible participants received OL GLM in period 1. In period 2, participants who achieved inactive disease were randomized 1:1:1 to receive double-blind (DB) treatment with monthly placebo (PBO, treatment withdrawal) or continued GLM treatment given monthly (GLM QMT) or every 2 months (GLM Q2MT). Participants who did not have a disease flare continued DB treatment for ∼12 months. Participants with a disease flare discontinued DB treatment and resumed monthly OL GLM. Primary endpoint compared the proportion of participants without a disease flare in the continued GLM treatment groups (QMT or Q2MT) vs PBO in a multiplicity-controlled, step-down fashion. Safety follow-up continued for ∼3 months after last treatment.
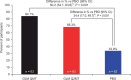
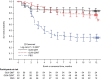
Results: A total of 188 patients, out of the 323 enrolled, were eligible for participation in period 2. Both GLM QMT and GLM Q2MT were superior to treatment withdrawal (PBO) in preventing disease flare (P < 0.001), with a treatment-difference vs PBO of 50.4% and 34.4% for the GLM QMT and GLM Q2MT groups, respectively. The time-to-first flare was significantly longer (log-rank P < 0.0001) with GLM treatment compared with PBO. Of 53 participants (in Q2MT or PBO) who had a confirmed disease flare, 51 (96.2%) attained a clinical response within 3 months of restarting OL GLM. Adverse events were consistent with the known GLM safety profile.
Conclusion: Among participants with active nr-axSpA who attained inactive disease after 10 months of GLM treatment, continued GLM treatment is well tolerated and provides superior protection against disease flares compared with GLM withdrawal. (EudraCT: 2015-004020-65, registered on 30 March 2022; NCT: 03253796, registered on 18 August 2017.).
Keywords: efficacy; golimumab; non-radiographic axial spondyloarthritis; reduced dosing; safety; withdrawal.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures


References
-
- Sieper J, Poddubnyy D.. Axial spondyloarthritis. Lancet 2017;390:73–84. - PubMed
-
- Ward MM, Deodhar A, Gensler LS. et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019;71:1599–613. - PMC - PubMed
-
- Ramiro S, Nikiphorou E, Sepriano A. et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis 2022;82:19–34. - PubMed
-
- Robinson PC, van der Linden S, Khan MA, Taylor WJ.. Axial spondyloarthritis: concept, construct, classification and implications for therapy. Nat Rev Rheumatol 2021;17:109–18. - PubMed
-
- van der Heijde D, Ramiro S, Landewé R. et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

