Development of Fluorescent 4-[4-(3 H-Spiro[isobenzofuran-1,4'-piperidin]-1'-yl)butyl]indolyl Derivatives as High-Affinity Probes to Enable the Study of σ Receptors via Fluorescence-Based Techniques
- PMID: 36919956
- PMCID: PMC10041534
- DOI: 10.1021/acs.jmedchem.2c01227
Development of Fluorescent 4-[4-(3 H-Spiro[isobenzofuran-1,4'-piperidin]-1'-yl)butyl]indolyl Derivatives as High-Affinity Probes to Enable the Study of σ Receptors via Fluorescence-Based Techniques
Abstract
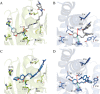
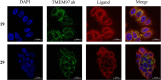
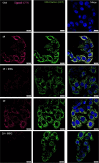
Sigma (σ) receptor subtypes, σ1 and σ2, are targets of wide pharmaceutical interest. The σ2 receptor holds promise for the development of diagnostics and therapeutics against cancer and Alzheimer's disease. Nevertheless, little is known about the mechanisms activated by the σ2 receptor. To contribute to the exploitation of its therapeutic potential, we developed novel specific fluorescent ligands. Indole derivatives bearing the N-butyl-3H-spiro[isobenzofuran-1,4'-piperidine] portion were functionalized with fluorescent tags. Nanomolar-affinity fluorescent σ ligands, spanning from green to red to near-infrared emission, were obtained. Compounds 19 (σ pan affinity) and 29 (σ2 selective), which displayed the best compromise between pharmacodynamic and photophysical properties, were investigated in flow cytometry, confocal, and live cell microscopy, demonstrating their specificity for the σ2 receptor. To the best of our knowledge, these are the first red-emitting fluorescent σ2 ligands, validated as powerful tools for the study of σ2 receptors via fluorescence-based techniques.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
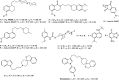
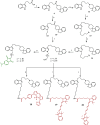
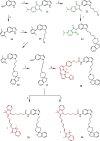
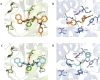



References
-
- Martin W. R.; Eades C. G.; Thompson J. A.; Huppler R. E.; Gilbert P. E. The effect of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information

