Excited-State Palladium-Catalyzed α-Selective C1-Ketonylation
- PMID: 36920159
- PMCID: PMC10010662
- DOI: 10.31635/ccschem.022.202202282
Excited-State Palladium-Catalyzed α-Selective C1-Ketonylation
Abstract
C-Glycosides are important carbohydrate mimetics found in natural products, bioactive compounds, and marketed drugs. However, stereoselective preparation of this class of glycomimetics remains a significant challenge in organic synthesis. Herein, we report an excited-state palladium-catalyzed α-selective C-ketonylation strategy using readily available 1-bromosugars to access a range of C-glycosides. The reaction features excellent α-selectivity and mild conditions that tolerate a wide range of functional groups and complex molecular architectures. The resulting α-ketonylsugars can serve as versatile precursors for their β-isomers and other C-glycosides. Preliminary experimental and computational studies of the mechanism suggest a radical pathway involving the formation of palladoradical and glycosyl radical that undergoes polarity-mismatched coupling with silyl enol ether, affording the desired α-ketonylsugars. Insight into the reactivity and mechanism will inspire new reaction development and provide straightforward access to both α- and β-C-glycosides, greatly expanding the chemical and patent spaces of glycomimetics.
Keywords: Excited-state catalysis; Palladium; glycosyl radical; ketonylation; silyl enol ethers.
Conflict of interest statement
Conflict of Interest There is no conflict of interest to report.
Figures
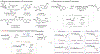

References
-
- Varki A; Cummings RD; Esko JD; Stanley P; Hart GW; Aebi M; Darvill AG; Kinoshita T; Packer NH; Prestegard JH; Schnaar RL; Seeberger PH, Essentials of Glycobiology. 3rd ed.; Cold Spring Harbor Press: Cold Spring Harbor, New York, 2017. - PubMed
-
- Yang Y; Yu B Recent advances in the chemical synthesis of C-glycosides. Chem. Rev 2017, 117, 12281–12356. - PubMed
-
- Wang H-Y; Blaszczyk SA; Xiao G; Tang W Chiral reagents in glycosylation and modification of carbohydrates. Chem. Soc. Rev 2018, 47, 681–701. - PubMed
-
- Nielsen MM; Pedersen CM Catalytic glycosylations in oligosaccharide synthesis. Chem. Rev 2018, 118, 8285–8358. - PubMed
-
- Kulkarni SS; Wang C-C; Sabbavarapu NM; Podilapu AR; Liao P-H; Hung S-C “One-pot” protection, glycosylation, and protection–glycosylation strategies of carbohydrates. Chem. Rev 2018, 118, 8025–8104. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
