Molecular insights into Escherichia coli Cpx envelope stress response activation by the sensor lipoprotein NlpE
- PMID: 36920223
- PMCID: PMC11391947
- DOI: 10.1111/mmi.15054
Molecular insights into Escherichia coli Cpx envelope stress response activation by the sensor lipoprotein NlpE
Abstract
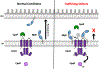
Bacterial two-component signal transduction systems provide sensory inputs for appropriately adapting gene expression. These systems rely on a histidine kinase that phosphorylates a response regulator which alters gene expression. Several two-component systems include additional sensory components that can activate the histidine kinase. In Escherichia coli, the lipoprotein NlpE was identified as a sensor for the Cpx cell envelope stress response. It has remained unclear how NlpE signals to Cpx in the periplasm. In this study, we used a combination of genetics, biochemistry, and AlphaFold2 complex modeling to uncover the molecular details of how NlpE triggers the Cpx response through an interaction with the CpxA histidine kinase. Remarkably, only a short loop of NlpE is required to activate the Cpx response. A single substitution in this loop inactivates NlpE signaling to Cpx and abolishes an in vivo biochemical NlpE:CpxA interaction. An independent AlphaFold multimer prediction supported a role for the loop and predicted an interaction interface at CpxA. Mutations in this CpxA region specifically blind the histidine kinase to NlpE activation but preserve the ability to respond to other cell envelope stressors. Hence, our work additionally reveals a previously unrecognized complexity in signal integration by the CpxA periplasmic sensor domain.
© 2023 John Wiley & Sons Ltd.
Conflict of interest statement
Figures
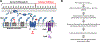

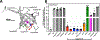

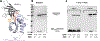
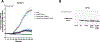
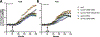
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

